The impact of isolated maternal hypothyroxinemia during the first and second trimester of gestation on pregnancy outcomes: an intervention and prospective cohort study in China
- PMID: 30334197
- PMCID: PMC6476837
- DOI: 10.1007/s40618-018-0960-7
The impact of isolated maternal hypothyroxinemia during the first and second trimester of gestation on pregnancy outcomes: an intervention and prospective cohort study in China
Abstract
Objectives: To explore the effect of isolated maternal hypothyroxinemia (IMH) during the first and second trimester of gestation on pregnancy outcomes. To explore whether levothyroxine (L-T4) treatment of women who had IMH identified in the first trimester improves pregnancy outcomes.
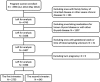
Methods: Women in the early pregnancy in the iodine-sufficient area (n = 3398) were recruited to this prospective cohort study (ChiCTR-TRC-12002326). Serum thyroid-stimulating hormone (TSH), free thyroxine (FT4), and thyroid peroxidase antibody (TPOAb) were detected. Women with IMH before 12 weeks chose to receive L-T4 or remain untreated. The L-T4 dose was adjusted to attain a normal FT4 and TSH level. Pregnancy outcomes were evaluated during follow-up.
Results: IMH in the first trimester was not associated with increased risk of adverse pregnancy outcome compared with controls. The incidence of macrosomia (p = 0.022) and gestational hypertension (p = 0.018) was significantly higher in IMH identified in the second trimester of gestation compared with controls. IMH identified in the second trimester of gestation was a risk factor for macrosomia [adjusted odds ratio (aOR) 1.942, 95% CI 1.076-3.503, p = 0.027] and gestational hypertension (aOR 4.203, 95% CI 1.611-10.968, p < 0.01), when body mass index in the early pregnancy was < 25 kg/m2.
Conclusions: IMH in the first trimester did not increase the risk of adverse outcomes irrespective of whether women received L-T4 treatment. However, IMH identified in the second trimester was associated with increased risk of adverse pregnancy outcome. The results suggest that thyroid function follow-up during the second trimester is necessary, even if thyroid function is normal during the first trimester.
Keywords: First and second trimester; Isolated maternal hypothyroxinemia (IMH); Levothyroxine (L-T4); Pregnancy outcomes.
Conflict of interest statement
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
The experimental procedure described here was approved by the Ethics Committee of the China Medical University ([2012] 2011-32-4) and is congruent with the Declaration of Helsinki.
Informed consent
Signed informed consent was obtained from every patient who participated.
References
MeSH terms
Substances
Grants and funding
- 2014BAI06B02/National Science and Technology Support Program
- 81170730/Chinese National Natural Science Foundation
- 81570709/Chinese National Natural Science Foundation
- 81500605/Chinese National Natural Science Foundation
- LZ2014035/Foundation of Key Laboratory in Department of Education of Liaoning Province Government in China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases