Vascular Biology of Superoxide-Generating NADPH Oxidase 5-Implications in Hypertension and Cardiovascular Disease
- PMID: 30334629
- PMCID: PMC6354601
- DOI: 10.1089/ars.2018.7583
Vascular Biology of Superoxide-Generating NADPH Oxidase 5-Implications in Hypertension and Cardiovascular Disease
Abstract
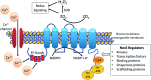
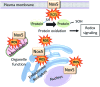
Significance: NADPH oxidases (Noxs), of which there are seven isoforms (Nox1-5, Duox1/Duox2), are professional oxidases functioning as reactive oxygen species (ROS)-generating enzymes. ROS are signaling molecules important in physiological processes. Increased ROS production and altered redox signaling in the vascular system have been implicated in the pathophysiology of cardiovascular diseases, including hypertension, and have been attributed, in part, to increased Nox activity. Recent Advances: Nox1, Nox2, Nox4, and Nox5 are expressed and functionally active in human vascular cells. While Nox1, Nox2, and Nox4 have been well characterized in models of cardiovascular disease, little is known about Nox5. This may relate to the lack of experimental models because rodents lack NOX5. However, recent studies have advanced the field by (i) elucidating mechanisms of Nox5 regulation, (ii) identifying Nox5 variants, (iii) characterizing Nox5 expression, and (iv) discovering the Nox5 crystal structure. Moreover, studies in human Nox5-expressing mice have highlighted a putative role for Nox5 in cardiovascular disease.
Critical issues: Although growing evidence indicates a role for Nox-derived ROS in cardiovascular (patho)physiology, the exact function of each isoform remains unclear. This is especially true for Nox5.
Future directions: Future directions should focus on clinically relevant studies to discover the functional significance of Noxs, and Nox5 in particular, in human health and disease. Two important recent studies will impact future directions. First, Nox5 is the first Nox to be crystallized. Second, a genome-wide association study identified Nox5 as a novel blood pressure-associated gene. These discoveries, together with advancements in Nox5 biology and biochemistry, will facilitate discovery of drugs that selectively target Noxs to interfere in uncontrolled ROS generation.
Keywords: Nox isoforms; atherosclerosis; endothelium; oxidative stress; reactive oxygen species; vascular smooth muscle.
Figures




Similar articles
-
NOX5: Molecular biology and pathophysiology.Exp Physiol. 2019 May;104(5):605-616. doi: 10.1113/EP086204. Epub 2019 Mar 18. Exp Physiol. 2019. PMID: 30801870 Free PMC article. Review.
-
NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function.J Am Heart Assoc. 2018 Jun 15;7(12):e009388. doi: 10.1161/JAHA.118.009388. J Am Heart Assoc. 2018. PMID: 29907654 Free PMC article.
-
NADPH oxidases in vascular pathology.Antioxid Redox Signal. 2014 Jun 10;20(17):2794-814. doi: 10.1089/ars.2013.5607. Epub 2013 Nov 1. Antioxid Redox Signal. 2014. PMID: 24180474 Free PMC article. Review.
-
The Molecular Regulation and Functional Roles of NOX5.Methods Mol Biol. 2019;1982:353-375. doi: 10.1007/978-1-4939-9424-3_22. Methods Mol Biol. 2019. PMID: 31172484 Review.
-
Central role of c-Src in NOX5- mediated redox signalling in vascular smooth muscle cells in human hypertension.Cardiovasc Res. 2022 Mar 25;118(5):1359-1373. doi: 10.1093/cvr/cvab171. Cardiovasc Res. 2022. PMID: 34320175 Free PMC article.
Cited by
-
Molecular Mechanism Underlying Role of the XBP1s in Cardiovascular Diseases.J Cardiovasc Dev Dis. 2022 Dec 14;9(12):459. doi: 10.3390/jcdd9120459. J Cardiovasc Dev Dis. 2022. PMID: 36547457 Free PMC article. Review.
-
Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease.J Transl Med. 2023 Aug 2;21(1):519. doi: 10.1186/s12967-023-04361-7. J Transl Med. 2023. PMID: 37533007 Free PMC article. Review.
-
GKT137831 and hydrogen peroxide increase the release of 6-nitrodopamine from the human umbilical artery, rat-isolated right atrium, and rat-isolated vas deferens.Front Pharmacol. 2024 Apr 5;15:1348876. doi: 10.3389/fphar.2024.1348876. eCollection 2024. Front Pharmacol. 2024. PMID: 38645555 Free PMC article.
-
NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors.J Med Chem. 2023 Sep 14;66(17):11632-11655. doi: 10.1021/acs.jmedchem.3c00770. Epub 2023 Aug 31. J Med Chem. 2023. PMID: 37650225 Free PMC article. Review.
-
Mechanistic computational modeling of the kinetics and regulation of NADPH oxidase 2 assembly and activation facilitating superoxide production.Free Radic Res. 2020 Oct;54(10):695-721. doi: 10.1080/10715762.2020.1836368. Epub 2020 Nov 9. Free Radic Res. 2020. PMID: 33059489 Free PMC article.
References
-
- Antony S, Jiang G, Wu Y, Meitzler JL, Makhlouf HR, Haines DC, Butcher D, Hoon DS, Ji J, Zhang Y, Juhasz A, Lu J, Liu H, Dahan I, Konate M, Roy KK, and Doroshow JH. NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates normoxic HIF-1α and p27Kip1 expression in malignant melanoma and other human tumors. Mol Carcinog 56: 2643–2662, 2017. - PMC - PubMed
-
- Bánfi B, Molnár G, Maturana A, Steger K, Hegedûs B, Demaurex N, and Krause KH. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276: 37594–37601, 2001 - PubMed
-
- Bánfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnár GZ, Krause KH, and Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279: 18583–18591, 2004 - PubMed
-
- Barman SA. and Fulton D. Adventitial fibroblast Nox4 expression and ROS signaling in pulmonary arterial hypertension. Adv Exp Med Biol 967: 1–11, 2017 - PubMed
-
- Bedard K, Jaquet V, and Krause KH. NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52: 725–734, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
