VX-659-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles
- PMID: 30334693
- PMCID: PMC6277022
- DOI: 10.1056/NEJMoa1807119
VX-659-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles
Abstract
Background: The next-generation cystic fibrosis transmembrane conductance regulator (CFTR) corrector VX-659, in triple combination with tezacaftor and ivacaftor (VX-659-tezacaftor-ivacaftor), was developed to restore the function of Phe508del CFTR protein in patients with cystic fibrosis.
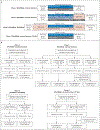
Methods: We evaluated the effects of VX-659-tezacaftor-ivacaftor on the processing, trafficking, and function of Phe508del CFTR protein using human bronchial epithelial cells. A range of oral VX-659-tezacaftor-ivacaftor doses in triple combination were then evaluated in randomized, controlled, double-blind, multicenter trials involving patients with cystic fibrosis who were heterozygous for the Phe508del CFTR mutation and a minimal-function CFTR mutation (Phe508del-MF genotypes) or homozygous for the Phe508del CFTR mutation (Phe508del-Phe508del genotype). The primary end points were safety and the absolute change from baseline in the percentage of predicted forced expiratory volume in 1 second (FEV1).
Results: VX-659-tezacaftor-ivacaftor significantly improved the processing and trafficking of Phe508del CFTR protein as well as chloride transport in vitro. In patients, VX-659-tezacaftor-ivacaftor had an acceptable safety and side-effect profile. Most adverse events were mild or moderate. VX-659-tezacaftor-ivacaftor resulted in significant mean increases in the percentage of predicted FEV1 through day 29 (P<0.001) of up to 13.3 points in patients with Phe508del-MF genotypes; in patients with the Phe508del-Phe508del genotype already receiving tezacaftor-ivacaftor, adding VX-659 resulted in a further 9.7-point increase in the percentage of predicted FEV1. The sweat chloride concentrations and scores on the respiratory domain of the Cystic Fibrosis Questionnaire-Revised improved in both patient populations.
Conclusions: Robust in vitro activity of VX-659-tezacaftor-ivacaftor targeting Phe508del CFTR protein translated into improvements for patients with Phe508del-MF or Phe508del-Phe508del genotypes. VX-659 triple-combination regimens have the potential to treat the underlying cause of disease in approximately 90% of patients with cystic fibrosis. (Funded by Vertex Pharmaceuticals; VX16-659-101 and VX16-659-001 ClinicalTrials.gov numbers, NCT03224351 and NCT03029455 .).
Figures


Comment in
-
Triple CFTR Modulator Therapy for Cystic Fibrosis.N Engl J Med. 2018 Oct 25;379(17):1671-1672. doi: 10.1056/NEJMe1811996. Epub 2018 Oct 18. N Engl J Med. 2018. PMID: 30334694 No abstract available.
-
Combination therapy.Nat Med. 2019 Jan;25(1):19. doi: 10.1038/s41591-018-0328-8. Nat Med. 2019. PMID: 30617328 No abstract available.
References
-
- The Clinical and Functional Translation of CFTR (CFTR2). Baltimore: Cystic Fibrosis Foundation, Johns Hopkins University, The Hospital for Sick Children, 2011. (https://www.cftr2.org).
-
- Elborn JS. Cystic fibrosis. Lancet 2016; 388: 2519–31. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989; 245: 1066–73. - PubMed
-
- Dalemans W, Barbry P, Champigny G, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 1991; 354: 526–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
