Diarrheal Etiology and Impact of Coinfections on Rotavirus Vaccine Efficacy Estimates in a Clinical Trial of a Monovalent Human-Bovine (116E) Oral Rotavirus Vaccine, Rotavac, India
- PMID: 30335135
- PMCID: PMC6603264
- DOI: 10.1093/cid/ciy896
Diarrheal Etiology and Impact of Coinfections on Rotavirus Vaccine Efficacy Estimates in a Clinical Trial of a Monovalent Human-Bovine (116E) Oral Rotavirus Vaccine, Rotavac, India
Abstract
Background: Rotavirus vaccine efficacy (VE) estimates in low-resource settings are lower than in developed countries. We detected coinfections in cases of severe rotavirus diarrhea in a rotavirus VE trial to determine whether these negatively impacted rotavirus VE estimates.
Methods: We performed TaqMan Array Card assays for enteropathogens on stools from rotavirus enzyme immunoassay-positive diarrhea episodes and all severe episodes (Vesikari score ≥11), from a phase 3 VE trial of Rotavac, a monovalent human-bovine (116E) rotavirus vaccine, carried out across 3 sites in India. We estimated pathogen-specific etiologies of diarrhea, described associated clinical characteristics, and estimated the impact of coinfections on rotavirus VE using a test-negative design.
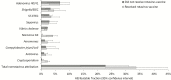
Results: A total of 1507 specimens from 1169 infants were tested for the presence of coinfections. Rotavirus was the leading cause of severe diarrhea even among vaccinated children, followed by adenovirus 40/41, Shigella/enteroinvasive Escherichia coli, norovirus GII, sapovirus, and Cryptosporidium species. Bacterial coinfections in rotavirus-positive diarrhea were associated with a longer duration of diarrhea and protozoal coinfections with increased odds of hospitalization. Using the test-negative design, rotavirus VE against severe rotavirus gastroenteritis increased from 49.3% to 60.6% in the absence of coinfections (difference, 11.3%; 95% confidence interval, -10.3% to 30.2%).
Conclusions: While rotavirus was the dominant etiology of severe diarrhea even in vaccinated children, a broad range of other etiologies was identified. Accounting for coinfections led to an 11.3% increase in the VE estimate. Although not statistically significant, an 11.3% decrease in VE due to presence of coinfections would explain an important fraction of the low rotavirus VE in this setting.
Keywords: attributable fraction; coinfection; diarrhea; rotavirus vaccine; vaccine efficacy.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Similar articles
-
Effect of Non-Rotavirus Enteric Infections on Vaccine Efficacy in a ROTASIIL Clinical Trial.Am J Trop Med Hyg. 2024 Apr 16;110(6):1201-1209. doi: 10.4269/ajtmh.23-0348. Print 2024 Jun 5. Am J Trop Med Hyg. 2024. PMID: 38626750 Free PMC article. Clinical Trial.
-
Evaluation of the Influence of Gastrointestinal Coinfections on Rotavirus Vaccine Effectiveness in Botswana.Pediatr Infect Dis J. 2018 Mar;37(3):e58-e62. doi: 10.1097/INF.0000000000001828. Pediatr Infect Dis J. 2018. PMID: 29189612 Free PMC article.
-
Effectiveness of Monovalent Rotavirus Vaccine After Programmatic Implementation in Botswana: A Multisite Prospective Case-Control Study.Clin Infect Dis. 2016 May 1;62 Suppl 2:S161-7. doi: 10.1093/cid/civ1207. Clin Infect Dis. 2016. PMID: 27059351
-
Rotavirus vaccines and vaccination in Latin America.Rev Panam Salud Publica. 2000 Nov;8(5):305-31. doi: 10.1590/s1020-49892000001000002. Rev Panam Salud Publica. 2000. PMID: 11190969 Review.
-
Epidemiology of rotavirus gastroenteritis and need of high rotavirus vaccine coverage with early completion of vaccination schedule for protection against rotavirus diarrhea in India: A narrative review.Indian J Public Health. 2019 Jul-Sep;63(3):243-250. doi: 10.4103/ijph.IJPH_307_18. Indian J Public Health. 2019. PMID: 31552856 Review.
Cited by
-
Sapovirus: an emerging cause of childhood diarrhea.Curr Opin Infect Dis. 2020 Oct;33(5):388-397. doi: 10.1097/QCO.0000000000000671. Curr Opin Infect Dis. 2020. PMID: 32796163 Free PMC article. Review.
-
Etiology and Incidence of Moderate-to-Severe Diarrhea in Young Children in Niger.J Pediatric Infect Dis Soc. 2021 Dec 31;10(12):1062-1070. doi: 10.1093/jpids/piab080. J Pediatric Infect Dis Soc. 2021. PMID: 34468743 Free PMC article. Clinical Trial.
-
Prevalence, Clinical Severity, and Seasonality of Adenovirus 40/41, Astrovirus, Sapovirus, and Rotavirus Among Young Children With Moderate-to-Severe Diarrhea: Results From the Vaccine Impact on Diarrhea in Africa (VIDA) Study.Clin Infect Dis. 2023 Apr 19;76(76 Suppl1):S123-S131. doi: 10.1093/cid/ciad060. Clin Infect Dis. 2023. PMID: 37074439 Free PMC article.
-
Molecular characterization of human astrovirus infection in children under 5 years of age with acute gastroenteritis in Tehran, Iran, 2021-2022: co-infection with rotavirus.Virus Genes. 2024 Aug;60(4):357-369. doi: 10.1007/s11262-024-02075-6. Epub 2024 May 15. Virus Genes. 2024. PMID: 38744749
-
Update on rotavirus vaccine underperformance in low- to middle-income countries and next-generation vaccines.Hum Vaccin Immunother. 2021 Jun 3;17(6):1787-1802. doi: 10.1080/21645515.2020.1844525. Epub 2020 Dec 17. Hum Vaccin Immunother. 2021. PMID: 33327868 Free PMC article. Review.
References
-
- Zaman K, Dang DA, Victor JC, et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. - PubMed
-
- Madhi SA, Cunliffe NA, Steele D, et al. . Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

