Genome Sequence of the Wheat Stem Sawfly, Cephus cinctus, Representing an Early-Branching Lineage of the Hymenoptera, Illuminates Evolution of Hymenopteran Chemoreceptors
- PMID: 30335145
- PMCID: PMC6250288
- DOI: 10.1093/gbe/evy232
Genome Sequence of the Wheat Stem Sawfly, Cephus cinctus, Representing an Early-Branching Lineage of the Hymenoptera, Illuminates Evolution of Hymenopteran Chemoreceptors
Abstract
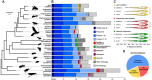
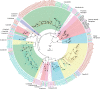
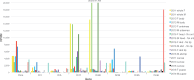
The wheat stem sawfly, Cephus cinctus, is a major pest of wheat and key ecological player in the grasslands of western North America. It also represents the distinctive Cephoidea superfamily of sawflies (Symphyta) that appeared early during the hymenopteran radiation, but after three early-branching eusymphytan superfamilies that form the base of the order Hymenoptera. We present a high-quality draft genome assembly of 162 Mb in 1,976 scaffolds with a scaffold N50 of 622 kb. Automated gene annotation identified 11,210 protein-coding gene models and 1,307 noncoding RNA models. Thirteen percent of the assembly consists of ∼58,000 transposable elements partitioned equally between Class-I and Class-II elements. Orthology analysis reveals that 86% of Cephus proteins have identifiable orthologs in other insects. Phylogenomic analysis of conserved subsets of these proteins supports the placement of the Cephoidea between the Eusymphyta and the parasitic woodwasp superfamily Orussoidea. Manual annotation and phylogenetic analysis of families of odorant, gustatory, and ionotropic receptors, plus odorant-binding proteins, shows that Cephus has representatives for most conserved and expanded gene lineages in the Apocrita (wasps, ants, and bees). Cephus has also maintained several insect gene lineages that have been lost from the Apocrita, most prominently the carbon dioxide receptor subfamily. Furthermore, Cephus encodes a few small lineage-specific chemoreceptor gene family expansions that might be involved in adaptations to new grasses including wheat. These comparative analyses identify gene family members likely to have been present in the hymenopteran ancestor and provide a new perspective on the evolution of the chemosensory gene repertoire.
Figures
Similar articles
-
Sawfly Genomes Reveal Evolutionary Acquisitions That Fostered the Mega-Radiation of Parasitoid and Eusocial Hymenoptera.Genome Biol Evol. 2020 Jul 1;12(7):1099-1188. doi: 10.1093/gbe/evaa106. Genome Biol Evol. 2020. PMID: 32442304 Free PMC article.
-
Odorant receptors of a primitive hymenopteran pest, the wheat stem sawfly.Insect Mol Biol. 2013 Dec;22(6):659-67. doi: 10.1111/imb.12053. Epub 2013 Aug 22. Insect Mol Biol. 2013. PMID: 23964849
-
Two nearly complete mitogenomes of wheat stem borers, Cephus pygmeus (L.) and Cephus sareptanus Dovnar-Zapolskij (Hymenoptera: Cephidae): an unusual elongation of rrnS gene.Gene. 2015 Mar 10;558(2):254-64. doi: 10.1016/j.gene.2014.12.069. Epub 2015 Jan 7. Gene. 2015. PMID: 25576223
-
Molecular evolution of the major chemosensory gene families in insects.Heredity (Edinb). 2009 Sep;103(3):208-16. doi: 10.1038/hdy.2009.55. Epub 2009 May 13. Heredity (Edinb). 2009. PMID: 19436326 Review.
-
Genomes of the Hymenoptera.Curr Opin Insect Sci. 2018 Feb;25:65-75. doi: 10.1016/j.cois.2017.11.008. Epub 2017 Nov 22. Curr Opin Insect Sci. 2018. PMID: 29602364 Free PMC article. Review.
Cited by
-
Chemoreceptor Diversity in Apoid Wasps and Its Reduction during the Evolution of the Pollen-Collecting Lifestyle of Bees (Hymenoptera: Apoidea).Genome Biol Evol. 2021 Mar 1;13(3):evaa269. doi: 10.1093/gbe/evaa269. Genome Biol Evol. 2021. PMID: 33484563 Free PMC article.
-
Sensory and behavioral responses of braconid parasitoids to changes in volatile emissions induced by wheat stem sawfly (Hymenoptera: Cephidae) larval feeding in winter wheat and smooth brome.J Insect Sci. 2025 Mar 14;25(2):2. doi: 10.1093/jisesa/ieaf016. J Insect Sci. 2025. PMID: 40085517 Free PMC article.
-
Genome-resolved insights into a novel Spiroplasma symbiont of the Wheat Stem Sawfly (Cephus cinctus).PeerJ. 2019 Aug 27;7:e7548. doi: 10.7717/peerj.7548. eCollection 2019. PeerJ. 2019. PMID: 31523509 Free PMC article.
-
Genome of the Parasitoid Wasp Diachasma alloeum, an Emerging Model for Ecological Speciation and Transitions to Asexual Reproduction.Genome Biol Evol. 2019 Oct 1;11(10):2767-2773. doi: 10.1093/gbe/evz205. Genome Biol Evol. 2019. PMID: 31553440 Free PMC article.
-
Genomes of the willow-galling sawflies Euura lappo and Eupontania aestiva (Hymenoptera: Tenthredinidae): a resource for research on ecological speciation, adaptation, and gall induction.G3 (Bethesda). 2021 May 7;11(5):jkab094. doi: 10.1093/g3journal/jkab094. G3 (Bethesda). 2021. PMID: 33788947 Free PMC article.
References
-
- Adhikari S, Seipel T, Menalled FD, Weaver DK.. 2018. Farming system and wheat cultivar affect infestation of and parasitism on Cephus cinctus in the Northern Great Plains. Pest Manag Sci. 74 (Early View):1–8. - PubMed
-
- Bartelt RJ, Cossé AA, Petroski RJ, Weaver DK.. 2002. Cuticular hydrocarbons and novel alkenediol iacetates from what stem sawfly (Cephus cinctus): natural oxidation to pheromone components. J Chem Ecol. 28(2):385–405. - PubMed
-
- Bekkerman A, Weaver DK.. 2018. Modeling joint dependence of managed ecosystems pests: the case of the wheat stem sawfly. J Agric Res Econ. 43:172–194.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

