Plasmodium falciparum-CD36 Structure-Function Relationships Defined by Ortholog Scanning Mutagenesis
- PMID: 30335152
- PMCID: PMC6386811
- DOI: 10.1093/infdis/jiy607
Plasmodium falciparum-CD36 Structure-Function Relationships Defined by Ortholog Scanning Mutagenesis
Abstract
Background: The interaction of Plasmodium falciparum-infected erythrocytes (IEs) with the host receptor CD36 is among the most studied host-parasite interfaces. CD36 is a scavenger receptor that binds numerous ligands including the cysteine-rich interdomain region (CIDR)α domains of the erythrocyte membrane protein 1 family (PfEMP1) expressed on the surface of IEs. CD36 is conserved across species, but orthologs display differential binding of IEs.
Methods: In this study, we exploited these differences, combined with the recent crystal structure and 3-dimensional modeling of CD36, to investigate malaria-CD36 structure-function relationships and further define IE-CD36 binding interactions.
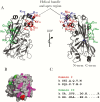
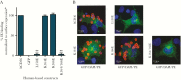
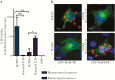
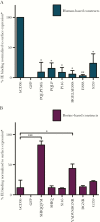
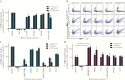
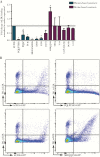
Results: We show that a charged surface in the membrane-distal region of CD36 is necessary for IE binding. Moreover, IE interaction with this binding surface is influenced by additional CD36 domains, both proximal to and at a distance from this site.
Conclusions: Our data indicate that subtle sequence and spatial differences in these domains modify receptor conformation and regulate the ability of CD36 to selectively interact with its diverse ligands.
Keywords: host-parasite interaction; infected erythrocytes; ortholog swap mutagenesis; scavenger receptor.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Modifications in the CD36 binding domain of the Plasmodium falciparum variant antigen are responsible for the inability of chondroitin sulfate A adherent parasites to bind CD36.Blood. 2001 May 15;97(10):3268-74. doi: 10.1182/blood.v97.10.3268. Blood. 2001. PMID: 11342458
-
CD36 peptides that block cytoadherence define the CD36 binding region for Plasmodium falciparum-infected erythrocytes.Blood. 1999 Sep 15;94(6):2121-7. Blood. 1999. PMID: 10477742
-
The C-terminal segment of the cysteine-rich interdomain of Plasmodium falciparum erythrocyte membrane protein 1 determines CD36 binding and elicits antibodies that inhibit adhesion of parasite-infected erythrocytes.Infect Immun. 2008 May;76(5):1837-47. doi: 10.1128/IAI.00480-07. Epub 2008 Feb 25. Infect Immun. 2008. PMID: 18299339 Free PMC article.
-
CD36 and malaria: friends or foes?Trends Parasitol. 2003 Oct;19(10):461-9. doi: 10.1016/j.pt.2003.08.006. Trends Parasitol. 2003. PMID: 14519584 Review. No abstract available.
-
PfEMP1 - A Parasite Protein Family of Key Importance in Plasmodium falciparum Malaria Immunity and Pathogenesis.Adv Parasitol. 2015 Apr;88:51-84. doi: 10.1016/bs.apar.2015.02.004. Epub 2015 Mar 23. Adv Parasitol. 2015. PMID: 25911365 Review.
Cited by
-
Role of CD36 in central nervous system diseases.Neural Regen Res. 2024 Mar;19(3):512-518. doi: 10.4103/1673-5374.380821. Neural Regen Res. 2024. PMID: 37721278 Free PMC article. Review.
-
Red Blood Cells: Chasing Interactions.Front Physiol. 2019 Jul 31;10:945. doi: 10.3389/fphys.2019.00945. eCollection 2019. Front Physiol. 2019. PMID: 31417415 Free PMC article. Review.
-
PSCAN: Spatial scan tests guided by protein structures improve complex disease gene discovery and signal variant detection.Genome Biol. 2020 Aug 26;21(1):217. doi: 10.1186/s13059-020-02121-0. Genome Biol. 2020. PMID: 32847609 Free PMC article.
-
Adhesion between P. falciparum infected erythrocytes and human endothelial receptors follows alternative binding dynamics under flow and febrile conditions.Sci Rep. 2020 Mar 11;10(1):4548. doi: 10.1038/s41598-020-61388-2. Sci Rep. 2020. PMID: 32161335 Free PMC article.
-
Hemozoin Promotes Lung Inflammation via Host Epithelial Activation.mBio. 2021 Feb 9;12(1):e02399-20. doi: 10.1128/mBio.02399-20. mBio. 2021. PMID: 33563839 Free PMC article.
References
-
- Murray CJ, Rosenfeld LC, Lim SS, et al. . Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 2012; 379:413–31. - PubMed
-
- World Health Organization. World Malaria Report 2016. Geneva: World Health Organization; 2016.
-
- Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol 2008; 62:445–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

