Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids
- PMID: 30335174
- PMCID: PMC6698951
- DOI: 10.1093/ntr/nty192
Formation of flavorant-propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids
Abstract
Introduction: "Vaping" electronic cigarettes (e-cigarettes) is increasingly popular with youth, driven by the wide range of available flavors, often created using flavor aldehydes. The objective of this study was to examine whether flavor aldehydes remain stable in e-cigarette liquids or whether they undergo chemical reactions, forming novel chemical species that may cause harm to the user.
Methods: Gas chromatography was used to determine concentrations of flavor aldehydes and reaction products in e-liquids and vapor generated from a commercial e-cigarette. Stability of the detected reaction products in aqueous media was monitored by ultraviolet spectroscopy and nuclear magnetic resonance spectroscopy, and their effects on irritant receptors determined by fluorescent calcium imaging in HEK-293T cells.
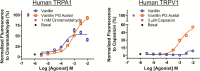
Results: Flavor aldehydes including benzaldehyde, cinnamaldehyde, citral, ethylvanillin, and vanillin rapidly reacted with the e-liquid solvent propylene glycol (PG) after mixing, and upward of 40% of flavor aldehyde content was converted to flavor aldehyde PG acetals, which were also detected in commercial e-liquids. Vaping experiments showed carryover rates of 50%-80% of acetals to e-cigarette vapor. Acetals remained stable in physiological aqueous solution, with half-lives above 36 hours, suggesting they persist when inhaled by the user. Acetals activated aldehyde-sensitive TRPA1 irritant receptors and aldehyde-insensitive TRPV1 irritant receptors.
Conclusions: E-liquids are potentially reactive chemical systems in which new compounds can form after mixing of constituents and during storage, as demonstrated here for flavor aldehyde PG acetals, with unexpected toxicological effects. For regulatory purposes, a rigorous process is advised to monitor the potentially changing composition of e-liquids and e-vapors over time, to identify possible health hazards.
Implications: This study demonstrates that e-cigarette liquids can be chemically unstable, with reactions occurring between flavorant and solvent components immediately after mixing at room temperature. The resulting compounds have toxicological properties that differ from either the flavorants or solvent components. These findings suggest that the reporting of manufacturing ingredients of e-liquids is insufficient for a safety assessment. The establishment of an analytical workflow to detect newly formed compounds in e-liquids and their potential toxicological effects is imperative for regulatory risk analysis.
© The Author(s) 2018. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Kinetics of Aldehyde Flavorant-Acetal Formation in E-Liquids with Different E-Cigarette Solvents and Common Additives Studied by 1H NMR Spectroscopy.Chem Res Toxicol. 2022 Aug 15;35(8):1410-1417. doi: 10.1021/acs.chemrestox.2c00159. Epub 2022 Jul 13. Chem Res Toxicol. 2022. PMID: 35830545 Free PMC article.
-
Chemical Adducts of Reactive Flavor Aldehydes Formed in E-Cigarette Liquids Are Cytotoxic and Inhibit Mitochondrial Function in Respiratory Epithelial Cells.Nicotine Tob Res. 2020 Dec 15;22(Suppl 1):S25-S34. doi: 10.1093/ntr/ntaa185. Nicotine Tob Res. 2020. PMID: 33320255 Free PMC article.
-
Effects of Common e-Liquid Flavorants and Added Nicotine on Toxicant Formation during Vaping Analyzed by 1H NMR Spectroscopy.Chem Res Toxicol. 2022 Jul 18;35(7):1267-1276. doi: 10.1021/acs.chemrestox.2c00110. Epub 2022 Jun 23. Chem Res Toxicol. 2022. PMID: 35735356 Free PMC article.
-
Toxicological and analytical assessment of e-cigarette refill components on airway epithelia.Sci Prog. 2016 Dec 1;99(4):351-398. doi: 10.3184/003685016X14773090197706. Sci Prog. 2016. PMID: 28742478 Free PMC article. Review.
-
Toxicology of flavoring- and cannabis-containing e-liquids used in electronic delivery systems.Pharmacol Ther. 2021 Aug;224:107838. doi: 10.1016/j.pharmthera.2021.107838. Epub 2021 Mar 18. Pharmacol Ther. 2021. PMID: 33746051 Free PMC article. Review.
Cited by
-
Kinetics of Aldehyde Flavorant-Acetal Formation in E-Liquids with Different E-Cigarette Solvents and Common Additives Studied by 1H NMR Spectroscopy.Chem Res Toxicol. 2022 Aug 15;35(8):1410-1417. doi: 10.1021/acs.chemrestox.2c00159. Epub 2022 Jul 13. Chem Res Toxicol. 2022. PMID: 35830545 Free PMC article.
-
Update on the Risks of Electronic Cigarettes-Vaping.Ochsner J. 2020 Spring;20(1):2-4. doi: 10.31486/toj.20.0012. Ochsner J. 2020. PMID: 32284674 Free PMC article. No abstract available.
-
The e-liquid flavoring cinnamaldehyde induces cellular stress responses in human proximal tubule (HK-2) kidney cells.Biomed Pharmacother. 2024 Jun;175:116666. doi: 10.1016/j.biopha.2024.116666. Epub 2024 Apr 27. Biomed Pharmacother. 2024. PMID: 38677246 Free PMC article.
-
Synthetic Cooling Agent in Oral Nicotine Pouch Products Marketed as "Flavor-Ban Approved".bioRxiv [Preprint]. 2023 Feb 24:2023.02.23.529797. doi: 10.1101/2023.02.23.529797. bioRxiv. 2023. Update in: Tob Control. 2025 Jan 2;34(1):106-110. doi: 10.1136/tc-2023-058035. PMID: 36865160 Free PMC article. Updated. Preprint.
-
The Untapped Biomarker Potential of MicroRNAs for Health Risk-Benefit Analysis of Vaping vs. Smoking.Cells. 2024 Aug 10;13(16):1330. doi: 10.3390/cells13161330. Cells. 2024. PMID: 39195220 Free PMC article. Review.
References
-
- Farsalinos KE, Poulas K, Voudris V, Le Houezec J. Electronic cigarette use in the European Union: analysis of a representative sample of 27460 Europeans from 28 countries. Addiction. 2016;111(11):2032–2040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

