Prokinetics for functional dyspepsia
- PMID: 30335201
- PMCID: PMC6516965
- DOI: 10.1002/14651858.CD009431.pub3
Prokinetics for functional dyspepsia
Abstract
Background: Dyspepsia is a common condition associated with gastrointestinal (GI) disease. Prokinetics are the treatment of choice for functional dyspepsia (FD). However, the role of prokinetics in FD treatment is still controversial.
Objectives: We conducted a systematic review and meta-analysis of randomised control trials (RCTs) examining the efficacy of prokinetics in the treatment of FD. The primary outcome was overall absence of or improvement of symptoms and symptom scores at the end of treatment. We also evaluated quality of life (QoL) and adverse events as secondary outcomes.
Search methods: We performed a systematic search of MEDLINE, Embase, the Cochrane Library, and CINAHL, from 1946 until September 2017. RevMan 5.3 was used to calculate pooled risk ratios (RR) of symptoms persisting or without improved QoL or adverse events, mean difference (MD) or standardised mean difference (SMD) of post-treatment symptoms scores, changes of symptom scores, and QoL, when appropriate with 95% confidence intervals (CI), using a random-effects model. Quality of evidence was evaluated using GRADE methodology.
Selection criteria: We included studies that were parallel group RCTs comparing one prokinetic with either placebo or another prokinetic of the same or different class for the treatment of FD. Studies involved adults who presented with dyspepsia symptoms and who had negative or insignificant findings on endoscopy as well as no other organic and metabolic disorders. Studies only including participants with primarily reflux or heartburn symptoms were excluded.
Data collection and analysis: Two review authors independently assessed study eligibility, study quality and performed data extraction.
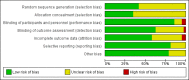
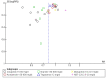
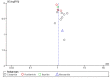
Main results: From an initial 1388 citations, we identified 43 studies in 40 papers. Of those, 29 studies with 10,044 participants compared six prokinetics with placebo for the outcome of absence of symptoms or symptom improvement. There was a statistically significant effect of prokinetic treatment in reducing global symptoms of FD (RR of remaining dyspeptic = 0.81, 95% CI 0.74 to 0.89; number needed to treat for an additional beneficial outcome (NNTB) =7, very low-quality evidence) with considerable heterogeneity; I2 = 91% (P < 0.00001). After removing cisapride from the analysis, the effect of prokinetics in global symptom improvement still persisted, compared to placebo (RR 0.87, 95% CI 0.80 to 0.94), but was still based on very low-quality evidence. The result showed persistence of significant improvement in subgroups of studies at unclear or at low risk of bias (RR 0.86, 95% CI 0.80-0.92), and in subgroups by molecules of cisapride (RR 0.71, 95% CI 0.54 to 0.93; NNTB = 4), acotiamide (RR 0.94, 95% CI 0.91 to 0.98; NNTB = 20) and tegaserod(RR 0.89, 95% CI 0.82 to 0.96; NNTB = 14).Ten studies compared different types of prokinetics with each other and the most commonly used comparator was domperidone, 10 mg three times a day (eight of the 10 studies). There was a significantly better post-treatment symptom score in other prokinetics, compared to domperidone (SMD -0.19, 95% CI -0.35 to -0.03, very low-quality evidence), but no difference in reducing global symptom (RR 0.94, 95% CI 0.83 to 1.07), and mean difference symptom scores (SMD -0.13, 95% CI -0.31 to 0.05). We found five studies that assessed quality of life, but there was no benefit in improving quality of life with prokinetic treatment (SMD 0.11, 95% CI -0.10 to 0.33; participants = 1774). The adverse events in individual prokinetics was not different from placebo (RR 1.09, 95% CI 0.95 to 1.25; participants = 3811; studies = 17). However, when we looked at the adverse effects by each prokinetic, there were overall greater adverse effects in the active treatment group with cisapride (RR 1.31, 95% CI 1.03 to 1.65; P = 0.03). The most common side effects were diarrhoea, abdominal discomfort and nausea. The funnel plot was asymmetric (Egger's test, P = 0.02) implying reporting bias or other small-study effects may be, in part, driving the benefit of prokinetics compared to placebo in this meta-analysis. The GRADE assessment of the quality of the evidence in each outcome are mostly low or very low due to concerns around risk of bias in study design, unexplained heterogeneity and possible publication bias.
Authors' conclusions: Due to low, or very low, quality of evidence, we are unable to say whether prokinetics are effective for the treatment of functional dyspepsia . We are uncertain which of the individual prokinetic drugs is the most effective as well as whether prokinetics can improve quality of life. Apart from cisapride, prokinetics are well-tolerated. Good quality RCTs are needed to verify the efficacy of prokinetics.
Conflict of interest statement
RP: none known. YY: none known. NB: has received speaker honoraria, consulting and reimbursement for expenses from AbbVie. RK: has received fees for consulting from Takeda, AbbVie, Jansen, Shire, Pfizer and Robarts Clinical Trials. GL: none known. PM: attends advisory board meetings of Allergan, and has received grants from Allergan and Takeda to support a research network evaluating irritable bowel syndrome and inflammatory bowel disease across Canada.
Figures

Update of
References
References to studies included in this review
Abid 2017 {published data only}
Al‐Quorain 1995 {published data only}
-
- Al‐Quorain A, Larbi EB, Al‐Shedoki F. A double‐blind, randomized, placebo‐controlled trial of cisapride in Saudi Arabs with functional dyspepsia. Scandavian Journal of Gastroenterology 1995;30:531‐4. - PubMed
Amarapurkar 2004 {published data only}
-
- Amarapurkar DN, Rane P. Randomised, double‐blind, comparative study to evaluate the efficacy and safety of ganaton (itopride hydrochloride) and mosapride citrate in the management of functional dyspepsia. Journal of the Indian Medical Association 2004;102(12):735‐7. - PubMed
Champion 1997 {published data only}
-
- Champion MC, MacCannell KL, Thomson AB, Tanton R, Eberhard S, Sullivan SN, et al. A double‐blind randomized study of cisapride in the treatment of nonulcer dyspepsia. Canadian Journal of Gastroenterology 1997;11(2):127‐34. - PubMed
Chen 2004 {published data only}
-
- Chen SY, Wang JY, Zhu CW, Yuan YZ, Zou B, Xia L, et al. A randomized controlled multi‐center clinical trial on mosapride in the treatment of functional dyspepsia. Zhonghua Liu Xing Bing Xue za Zhi 2004;25(2):165‐8. - PubMed
Choi 2015 {published data only}
de Groot 1997 {published data only}
-
- Groot GH, Both PS. Cisapride in functional dyspepsia in general practice. A placebo‐controlled, randomized, double‐blind study. Alimentary Pharmacology & Therapeutics 1997;11(1):193‐9. - PubMed
De Nutte 1989 {published data only}
-
- Nutte N, Ganse W, Witterhulghe M, Defrance P. Relief of epigastric pain in nonulcer dyspepsia: controlled trial of the promotility drug cisapride. Clinical Therapeutics 1989;11(1):62‐8. - PubMed
Du 2014 {published data only}
-
- Du Y, Su T, Song X, Gao J, Zou D, Zuo C, et al. Efficacy and safety of cinitapride in the treatment of mild to moderate postprandial distress syndrome‐predominant functional dyspepsia. Journal of Clinical Gastroenterology 2014;48(4):328‐35. - PubMed
Francois 1987 {published data only}
-
- Francois I, Nutte N. Nonulcer dyspepsia: effect of the gastrointestinal prokinetic drug cisapride. Current Therapeutic Research 1987;41(6):891‐8.
Hallerback 2002 {published data only}
-
- Hallerback BI, Bommelaer G, Bredberg E, Campbell M, HellblomM, Lauritsen K, et al. Dose finding study of mosapride in functional dyspepsia: a placebo‐controlled, randomized study. Alimentary Pharmacology & Therapeutics 2002;16:959‐67. - PubMed
Hansen 1998 {published data only}
-
- Hansen JM, Bytzer P, Schaffalitzky de Muckadell OB. Placebo‐controlled trial of cisapride and nizatidine in unselected patients with functional dyspepsia. American Journal of Gastroenterology 1998;93(3):368‐74. - PubMed
Holtmann 2002 {published data only}
-
- Holtmann G, Gschossmann J, Mayr P, Talley NJ. A randomized placebo‐controlled trial of simethicone and cisapride for the treatment of patients with functional dyspepsia. Alimentary Pharmacology & Therapeutics 2002;16:1641‐8. - PubMed
Holtmann 2006 {published data only}
-
- Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo‐controlled trial of itopride in functional dyspepsia. New England Journal of Medicine 2006;354(8):832‐40. - PubMed
Jian 1989 {published data only}
-
- Jian R, Ducrot F, Ruskone A, Chaussade S, Rambaud JC, Modiglian R, et al. Symptomatic, radionuclide and therapeutic assessment of chronic idiopathic dyspepsia. A double‐blind placebo‐controlled evaluation of cisapride. Digestive Diseases and Sciences 1989;34(5):657‐64. - PubMed
Kellow 1995 {published data only}
-
- Kellow JE, Cowan H, Shuer B, Riley JW, Lunzer MR, Eckstein RP, et al. Efficacy of cisapride therapy in functional dyspepsia. Alimentary Pharmacology & Therapeutics 1995;9:153‐60. - PubMed
Kusunoki 2012 {published data only}
-
- Kusunoki H, Haruma K, Manabe N, Imamura H, Kamada T, Shiotani A, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real‐time ultrasonography. Neurogastroenterology and Motility 2012;24(6):540–5. - PubMed
Li 2005 {published data only}
-
- Li YH, Gong PL, Hou XH, Chen J, Liu NZ, Tian DA, et al. Itopride in treatment of 104 patients with functional dyspepsia: a randomized, double blind controlled clinical trial. Zhongguo Xinyao Yu Linchuang Zazhi 2005;7:524‐8.
Lin 2009 {published data only}
-
- Lin J, Ren M, Peng X, Xiao Y, Wang S, Yang L, et al. Short‐term efficacy of mosapride dispersible tablet on postprandial distress syndrome [莫沙必利分散片治疗餐后不适综合征的近期疗效观察]. 胃肠病学 (Chinese Journal of Gastrenterology) 2009;14(8):488–90.
Ma 2012 {published data only}
-
- Ma TT, Yu SY, Li Y, Liang FR, Tian XP, Zheng H, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Alimentary Pharmacology & Therapeutics 2012;35:552–61. - PubMed
Matsueda 2010a {published data only}
-
- Matsueda K, Hongo M, Sasaki D, Kusano M, Harasawa S, Arakawa T, et al. Therapeutic efficacy of novel agent (Z‐338) in functional dyspepsia (FD). Gastroenterology 2005;128 (supplement1):A467.
-
- Matsueda K, Hongo M, Tack J, Aoki H, Saito Y, Kato H. Clinical trial: dose‐dependent therapeutic efficacy of acotiamide hydrochloride (Z‐338) in patients with functional dyspepsia ‐ 100mg tid is an optimal dosage. Neurogastroenterology and Motility 2010;22:618‐e173. - PubMed
Matsueda 2010b {published data only}
-
- Matsueda K, Hongo M, Tack J, Aoki H, Saito Y, Kato H. Clinical trial: dose‐dependent therapeutic efficacy of acotiamide hydrochloride (Z‐338) in patients with functional dyspepsia ‐ 100mg tid is an optimal dosage. Neurogastroenterology and Motility 2010;22:618‐e173. - PubMed
Matsueda 2012 {published data only}
Mo 2003 {published data only}
-
- Mo JZ, Li DG, Jiang JH, Jiang YB, Wang XP, Gong ZH, et al. A multi‐center clinical trial of itopride hydrochloride in the treatment of functional dyspepsia [盐酸伊托必利治疗功能性消化不良的多中心临床研究]. 中国新药杂志 Zhongguo Xinyao Zazhi (Chinese Journal of New Drugs) 2003;12(6):467‐9.
Nakamura 2017 {published data only}
-
- Nakamura K, Tomita T, Oshima T, Asano H, Yamasaki T, Okugawa T, et al. A double‐blind placebo controlled study of acotiamide hydrochloride for efficacy on gastrointestinal motility of patients with functional dyspepsia. Journal of Gastroenterology 2017;52(5):602‐10. - PubMed
Rösch 1987 {published data only}
-
- Rösch W. Cisapride in non‐ulcer dyspepsia. Results of a placebo‐controlled trial. Scandinavian Journal of Gastroenterology 1987;22(2):161‐4. - PubMed
Shen 2014 {published data only}
-
- Shen Y, Fang N, Yang C‐H. Azintamide combined with itopride hydrochloride for treatment of functional dyspepsia [联合治疗功能性消化不良患者的临床疗效]. Shi Jie Wei Chang Bing Xue Za Zhi She [World Chinese Journal of Digestology] 2014;22(20):2993‐6.
Singh 2015 {published data only}
Sun 2003 {published data only}
-
- Sun J, Zhang CL, Chu Y, Yuan YZ, Li ZS, Liu XG, et al. A multi‐center, double‐blind, randomized and controlled trial of itopride hydrochloride in treatment of functional dyspepsia [伊托必利治疗功能性消化不良多中心、随机、双盲、双模拟临床研究]. 上海医学 [Shanghai Medical Journal] 2003;26(4):227‐9.
Tack 2009 {published data only}
-
- Tack J, Masclee A, Heading R, Berstad A, Piessevaux H, Popiela T, et al. A dose‐ranging, placebo‐controlled, pilot trial of Acotiamide in patients with functional dyspepsia. Neurogastroenterology and Motility 2009;21:272‐80. - PubMed
Tack 2011 {published data only}
-
- Tack JF, Stanghellini V, Holtmann G, Varannes SB, Vandenberghe A, Morooka K, et al. Efficacy and safety study of acotiamide (Z‐338) in European patients with functional dyspepsia. Gastroenterology 2011;140:S805.
Talley 2000 {published data only}
-
- Talley NJ, Verlinden M, Snape W, Beker JA, Ducrotte P, Dettmer A, et al. Failure of a motilin receptor agonist (ABT‐229) to relieve the symptoms of functional dyspepsia in patients with an without, delayed gastric emptying: a randomized double‐blind placebo‐controlled trial. Alimentary Pharmacology & Therapeutics 2000;14(12):1653‐61. - PubMed
Talley 2008a {published data only}
-
- Talley NJ, Tack JF, Kowalski DL, Borton MA, Barve A. A novel acetylcholine esterase inhibitor acotiamide hydrochloride (YM443) in functional dyspepsia: efficacy in a randomized, double‐blind, placebo controlled dose ranging trial. Gastroenterology 2008;134(4 Suppl 1):A‐157.
Talley 2008b {published data only}
-
- Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double‐blind, placebo‐controlled trials. Gut 2008;57(6):740‐6. - PubMed
Talley 2008c {published data only}
-
- Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double‐blind, placebo‐controlled trials. Gut 2008;57(6):740‐6. - PubMed
Teixeira 2000 {published data only}
-
- Teixeira CR, Abdalla Kurban AC, Denicol IP, Vieira Coelho NH, Spinato Torresini RJ, Peter RB, et al. Randomized, double‐blind study of functional dyspepsia with cisapride, trimebutine and placebo ‐ relationship with variation of the gastric emptying time. Revista Brasileira de Medicina 2000;57:729‐35.
Vakil 2008a {published data only}
-
- Vakil N, Laine L, Talley NJ, Zakko SF, Tack J, Chey WD, et al. Tegaserod treatment for dysmotility‐like functional dyspepsia: results of two randomized, controlled trials. American Journal of Gastroenterology 2008;103(8):1906–19. - PubMed
Vakil 2008b {published data only}
-
- Vakil N, Laine L, Talley NJ, Zakko SF, Tack J, Chey WD, et al. Tegaserod treatment for dysmotility‐like functional dyspepsia: results of two randomized, controlled trials. American Journal of Gastroenterology 2008;103(8):1906–19. - PubMed
Wang 1995 {published data only}
-
- Wang B, Liang X, Jia B, Jia B. A controlled multi‐centre clinical trial on cisapride in treatment of functional dyspepsia [西沙必利治疗功能性消化不良的多中心临床疗效观察]. Zhonghua Nei Ke za Zhi [Chinese Journal of Internal Medicine] 1995;34(3):180‐4. - PubMed
Wong 2014 {published data only}
-
- Wong Z, Nadirah Daud U, Naidu J, Soon Ngiu C, Affendi Raja Ali R, Palaniappan S, et al. Randomised, double‐blind placebo controlled trial assessing the efficacy of itopride in postprandial distress syndrome (PDS): a pilot study: P‐215. Journal of Gastroenterology and Hepatology 2014;29:124‐5.
Yeoh 1997 {published data only}
-
- Yeoh KG, Kang JY, Tay HH, Gwee KA, Tan CC, Wee A, et al. Effect of cisapride on functional dyspepsia in patients with an without histological gastritis: A double‐blind placebo‐controlled trial. Journal of Gastroenterology and Hepatology 1997;12(1):13‐8. - PubMed
Zhou 2000 {published data only}
-
- Yuan YZ, Zhou LY, Li BQ, Lin SR, Wang AY, Dong XY, et al. A multicenter clinical trial on itopride hydrochloride for treatment of functional dyspepsia [(盐酸伊托必利治疗功能性消化不良的多中心临床研究]. 中国临床药理学杂志 Zhongguo Linchuang Yaolixue Zazhi [Chinese Journal of Clinical Pharmacology] 2000;16(6):403‐7.
Zhu 2005 {published data only}
-
- Zhu CQ, Mao YM, Zeng MD, Dong SX, Xu GM, Wang GS, et al. A clinical study of hydrochloride itopride in the treatment of functional dyspepsia. Zhongguo Yaoke Daxue Xuebao [Journal of China Pharmaceutical University] 2005;6:580‐3.
References to studies excluded from this review
Agorastos 1991 {published data only}
-
- Agorastos I, Akriviadis E, Goulis G. Effect of cisapride in nonulcer dyspepsia: A placebo‐controlled trial. Current Therapeutic Research, Clinical and Experimental 1991;49(5):870‐7.
Bekhti 1979 {published data only}
-
- Bekhti A, Rutgeerts L. Domperidone in the treatment of functional dyspepsia in patients with delayed gastric emptying. Postgraduate Medical Journal 1979;55(Suppl 1):30‐2. - PubMed
Chen 2010 {published data only}
Chey1982 {published data only}
-
- Chey WY, You CH, Ange DA. Open and double blind clinical trials of domperidone in patients with unexplained nausea, vomiting, abdominal bloating and early satiety. Gastroenterology 1982;82(5):1033.
Chung1993 {published data only}
-
- Chung JM. Cisapride in chronic dyspepsia: results of a double‐blind, placebo‐controlled trial. Scandinavian Journal of Gastroenterology 1993;28(9):11‐4. - PubMed
Creytens 1984 {published data only}
-
- Creytens G. Effect of the non‐antidopaminergic drug cisapride on postprandial nausea. Current Therapeutic Research, Clinical and Experimental 1984;36(5II):1063‐70.
Davis1988 {published data only}
-
- Davis RH, Clench MH, Mathias JR. Effects of domperidone in patients with chronic unexplained upper gastrointestinal symptoms: a double‐blind,placebo‐controlled study. Digestive Diseases and Sciences 1988;33(12):1501‐11. - PubMed
De Loose 1979 {published data only}
-
- Loose F. Domperidone in chronic dyspepsia: a pilot open study and a multicentre general practice crossover comparison with metoclopramide and placebo. Pharmatherapeutica 1979;2:140‐6.
Deruyttere 1987 {published data only}
-
- Deruyttere M, Lepoutre L, Heyen H, Samain H, Pennoit H. Cisapride in the management of chronic functional dyspepsia: a multicenter, double‐blind, placebo‐controlled study. Clinical Therapeutics 1987;10(1):44‐51. - PubMed
Goethals 1987 {published data only}
-
- Goethals C, Mierop L. Cisapride in the treatment of chronic functional dyspepsia. Current Therapeutic Research, Clinical and Experimental 1987;42(2):261‐7.
Haarmann 1979 {published data only}
-
- Haarmann K, Lebkuchner F, Widmann A, Kief W, Esslinger M. A double‐blind study of domperidone in the symptomatic treatment of chronic post‐prandial upper gastrointestinal distress. Postgraduate Medical Journal 1979;55(Suppl 1):24‐7. - PubMed
Hannon 1987 {published data only}
-
- Hannon R. Efficacy of cisapride in patients with nonulcer dyspepsia. Current Therapeutic Research 1987;42(5):814‐22.
Hausken 1992 {published data only}
-
- Hausken T, Berstad A. Cisapride treatment of patients with non‐ulcer dyspepsia and erosive prepyloric changes. A double‐blind, placebo‐controlled trial. Scandinavian Journal of Gastroenterology 1992;27(3):213‐7. - PubMed
Kas'ianenko 2014 {published data only}
-
- Kas'ianenko VI, Denisov NL, Vasil'ev IV. Use of itopride in the symptoms of functional dyspepsia in Russia: results of a phase IV prospective open‐label multicenter clinical trial. Terapevticheskii Arkhiv 2014;86(8):35‐41. - PubMed
Kearney 2000 {published data only}
-
- Kearney DJ, Avins AL, McQuaid KR. Treatment of uninvestigated dyspepsia with cisapride for patients with negative Helicobacter pylori serologies. American Journal of Gastroenterology 2000;95(9):2212‐17. - PubMed
Kim 2010 {published data only}
-
- Kim YM, Park YC, Jo JH, Kang WC, Son MW, Hong KE. Effect of herb medicine treatment for functional dyspepsia: a randomized placebo‐controlled and compared standard treatment trial. Journal of Korean Medicine 2010;31(1):1‐13.
Liu 2013 {published data only}
-
- Liu B, Piao X, Guo L. Effect of herbal formula xiao pi‐II on functional dyspepsia. Journal of Traditional Chinese Medicine / Chung i Tsa Chih Ying Wen Pan 2013;33(3):298‐302. - PubMed
Manayagi 2014 {published data only}
-
- Mayanagi S, Kishino M, Kitagawa Y, Sunamura M. Efficacy of acotiamide in combination with esomeprazole for functional dyspepsia refractory to proton‐pump inhibitor monotherapy. Tohoku Journal of Experimental Medicine 2014;234(4):237‐40. - PubMed
Milo 1984 {published data only}
-
- Milo R. Non‐cholinergic, non‐antidopaminergic treatment of chronic digestive symptoms suggestive of a motility disorder: a two‐step pilot evaluation of cisapride. Current Therapeutic Research, Clinical and Experimental 1984;36:1053‐62.
Miwa 2009 {published data only}
-
- Miwa H, Nagahara A, Tominaga K, Yokoyama T, Sawada Y, Inoue K, et al. Efficacy of the 5‐HT1A agonist tandospirone citrate in improving symptoms of patients with functional dyspepsia: a randomized controlled trial. American Journal of Gastroenterology 2009;104:2779‐87. - PubMed
Shim 2015 {published data only}
-
- Shim YK, Lee JY, Kim NY, Park YH, Yoon H, Shin CM, et al. Efficacy and safety of new prokinetic agent benachio Q solution in patients with postprandial distress syndrome subtype in functional dyspepsia: a single‐center, randomized, double‐blind, placebo‐controlled pilot study. Taehan Sohwagi Hakhoe Chi [Korean Journal of Gastroenterology] 2015;66(1):17‐26. - PubMed
Tack 2012 {published data only}
-
- Tack J, Janssen P, Masaoka T, Farré R, Oudenhove L. Efficacy of buspirone, a fundus‐relaxing drug, in patients with functional dyspepsia. Clinical Gastroenterology & Hepatology 2012;10(11):1239‐45. - PubMed
Tack 2016 {published data only}
-
- Tack J, Rotondo A, Meulemans A, Thielemans L, Cools M. Randomized clinical trial: A controlled pilot trial of the 5‐HT4 receptor agonist revexepride in patients with symptoms suggestive of gastroparesis. Neurogastroenterology and Motility 2016;28(4):487‐97. - PubMed
Talley 2001 {published data only}
-
- Talley NJ, Zanten SV, Saez LR, Dukes G, PerschyT, Heath M, et al. A dose‐ranging, placebo‐controlled, randomized trial of alosetron in patients with functional dyspepsia. Alimentary Pharmacology & Therapeutics 2001;15:525‐37. - PubMed
Testoni 1990 {published data only}
-
- Testoni PA, Masci E, Bagnolo F, Passaretti S, Pellegrini A, Ronchi G, et al. Effect of long‐term oral therapy with cisapride in reflux antral gastritis: results of a double‐blind placebo‐controlled trial. Current Therapeutic Research 1990;47(1):156‐65.
Van de Mierop 1979 {published data only}
-
- Mierop L, Rutgeerts L, Langenbergh B, Staessen A. Oral domperidone in chronic postprandial dyspepsia. Digestion 1979;19(4):244‐50. - PubMed
Van Ganse 1978 {published data only}
-
- Ganse W, Damme L, Mierop L, Deruyttere M, Lauwers W, Coegegrachts J. Chronic dyspepsia: double‐blind treatment with domperidone (R 33812) or a placebo. A multicentre therapeutic evaluation. Current Therapeutic Research, Clinical and Experimental 1978;23(11):695‐701.
Van Outryve M {published data only}
-
- Outryve M, Lauwers W, Verbeke S. Domperidone for the symptomatic treatment of chronic post‐prandial nausea and vomiting.. Postgraduate Medical Journal 1979;55(Suppl 1):33‐5. - PubMed
Wood 1993 {published data only}
-
- Wood SF, Penney SC, Cochran KM. Cisapride in functional dyspepsia: a double‐blind, placebo‐controlled randomized trial in general practice patientsFunctional Dyspepsia: A Double‐Blind, Placebo‐Controlled Randomized Trial in General Practice Patients. Scandinavian Journal of Gastroenterology. Supplement 1993;28(195):5‐10. - PubMed
Yamawaki 2016 {published data only}
-
- Yamawaki H, Futagami S, Kawagoe T, Maruki Y, Hashimoto S, Nagoya H, et al. Improvement of meal‐related symptoms and epigastric pain in patients with functional dyspepsia treated with acotiamide was associated with acylated ghrelin levels in Japan. Neurogastroenterology and Motility 2016;28(7):1037‐47. - PubMed
Yan 2012 {published data only}
-
- Yan X, Feng C, Liu Z, Ji J, Chen S. A randomized, double‐blinded, placebo‐controlled, parallel study on efficacy of mosapride on nocturnal dyspeptic symptoms. Chinese Journal of Gastroenterology 2012;17(3):164‐7.
Additional references
Deeks 2011
-
- Deeks JJ, Higgins JPT, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group, editors. Chapter 9.4: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Egger 1997
Ford 2010
-
- Ford AC, Marwaha A, Lim A, Moayyedi P. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta‐analysis. Clinical Gastroenterology and Hepatology 2010;8:830‐7. - PubMed
Ford 2015
-
- Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta‐analysis. Gut 2015;64(7):1049–57. - PubMed
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed prior to April 22, 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].The Cochrane Collaboration, 2011. .Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group, editors. Chapter 16.1.3.2: Imputing standard deviations for changes from baseline. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lacy 2012
Lacy 2013
-
- Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Alimentary Pharmacology & Therapeutics 2013;38:170‐7. - PubMed
Loannidis 2007
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. - PubMed
Moayyedi 2002
Moayyedi 2011
-
- Moayyedi P, Soo S, Deeks JJ, Delaney B, Innes M, Forman D. Pharmacological interventions for non‐ulcer dyspepsia. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD001960.pub4] - DOI
Moayyedi 2017
Stanghellini 2016
-
- Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380‐92. - PubMed
Tack 2004
-
- Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology 2004;127:1239‐55. - PubMed
Tack 2006
-
- Tack J, Talley MJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, et al. Functional gastroduodenal disorders. Gastroenterology 2006;130:1466‐79. - PubMed
Talley 1991
-
- Talley NJ, Colin‐Jones DG, Koch KL, Koch M, Nyren O, Stanghellini V. Functional dyspepsia: a classification with guidelines for diagnosis and management. Gastroenterology International 1991;4:145‐60.
Tally 1999
Tally 2005
-
- Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology 2005;129:1756‐80. - PubMed
van Zanten 2011
-
- Zanten SV, Wahlqvist P, Talley NJ, Halling K, Vakil N, Lauritsen K, et al. Randomised clinical trial: the burden of illness of uninvestigated dyspepsia before and after treatment with esomeprazole—results from the STARS II study. Alimentary Pharmacology & Therapeutics 2011;34(7):714‐23. - PubMed
Vanheel 2013
-
- Vanheel H, Farré R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nature Reviews. Gastroenterology & Hepatology 2013;10:142–9. - PubMed
References to other published versions of this review
Bollegala 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

