Effects of reward on spatial working memory in schizophrenia
- PMID: 30335439
- PMCID: PMC6197071
- DOI: 10.1037/abn0000369
Effects of reward on spatial working memory in schizophrenia
Abstract
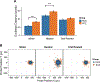
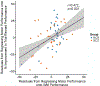
Reward processing and cognition are disrupted in schizophrenia (SCZ), yet how these processes interface is unknown. In SCZ, deficits in reward representation may affect motivated, goal-directed behaviors. To test this, we examined the effects of monetary reward on spatial working memory (WM) performance in patients with SCZ. To capture complimentary effects, we tested biophysically grounded computational models of neuropharmacologic manipulations onto a canonical fronto-parietal association cortical microcircuit capable of WM computations. Patients with SCZ (n = 33) and healthy control subjects (HCS; n = 32) performed a spatial WM task with 2 reward manipulations: reward cues presented prior to each trial, or contextually prior to a block of trials. WM performance was compared with cortical circuit models of WM subjected to feed-forward glutamatergic excitation, feed-forward GABAergic inhibition, or recurrent modulation strengthening local connections. Results demonstrated that both groups improved WM performance to reward cues presented prior to each trial (HCS d = -0.62; SCZ d = -1.0), with percent improvement correlating with baseline WM performance (r = .472, p < .001). However, rewards presented contextually before a block of trials did not improve WM performance in patients with SCZ (d = 0.01). Modeling simulations achieved improved WM precision through strengthened local connections via neuromodulation, or feed-forward inhibition. Taken together, this work demonstrates that patients with SCZ can improve WM performance to short-term, but not longer-term rewards-thus, motivated behaviors may be limited by strength of reward representation. A potential mechanism for transiently improved WM performance may be strengthening of local fronto-parietal microcircuit connections via neuromodulation or feed-forward inhibitory drive. (PsycINFO Database Record (c) 2018 APA, all rights reserved).
Figures



Similar articles
-
Schizophrenia is associated with a pattern of spatial working memory deficits consistent with cortical disinhibition.Schizophr Res. 2017 Mar;181:107-116. doi: 10.1016/j.schres.2016.10.011. Epub 2016 Oct 10. Schizophr Res. 2017. PMID: 27745755 Free PMC article.
-
Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory.Schizophr Res. 2010 Aug;121(1-3):146-52. doi: 10.1016/j.schres.2010.05.023. Epub 2010 Jul 3. Schizophr Res. 2010. PMID: 20598857
-
Targeting Frontal Gamma Activity with Neurofeedback to Improve Working Memory in Schizophrenia.Curr Top Behav Neurosci. 2023;63:153-172. doi: 10.1007/7854_2022_377. Curr Top Behav Neurosci. 2023. PMID: 35989397 Review.
-
Negative and nonemotional interference with visual working memory in schizophrenia.Biol Psychiatry. 2011 Dec 15;70(12):1159-68. doi: 10.1016/j.biopsych.2011.07.010. Epub 2011 Aug 20. Biol Psychiatry. 2011. PMID: 21861986
-
Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia.Biol Psychiatry. 2015 Jun 15;77(12):1010-9. doi: 10.1016/j.biopsych.2015.02.034. Epub 2015 Mar 4. Biol Psychiatry. 2015. PMID: 25847179 Review.
Cited by
-
The effect of reward expectation on working memory of emotional faces under different levels of cognitive load: an ERP study.Exp Brain Res. 2024 Mar;242(3):769-780. doi: 10.1007/s00221-023-06776-6. Epub 2024 Feb 3. Exp Brain Res. 2024. PMID: 38310175
-
Trying Harder: How Cognitive Effort Sculpts Neural Representations during Working Memory.J Neurosci. 2024 Jul 10;44(28):e0060242024. doi: 10.1523/JNEUROSCI.0060-24.2024. J Neurosci. 2024. PMID: 38769009 Free PMC article.
-
Nonsocial and social cognition in schizophrenia: current evidence and future directions.World Psychiatry. 2019 Jun;18(2):146-161. doi: 10.1002/wps.20624. World Psychiatry. 2019. PMID: 31059632 Free PMC article.
-
Effects of neuroactive metabolites of the tryptophan pathway on working memory and cortical thickness in schizophrenia.Transl Psychiatry. 2021 Apr 1;11(1):198. doi: 10.1038/s41398-021-01311-z. Transl Psychiatry. 2021. PMID: 33795641 Free PMC article.
-
A Translational Neuroscience & Computational Evaluation of a D1R Partial Agonist for Schizophrenia (TRANSCENDS): Rationale and Study Design of a Brain-Based Clinical Trial.medRxiv [Preprint]. 2025 Apr 20:2025.04.18.25326082. doi: 10.1101/2025.04.18.25326082. medRxiv. 2025. PMID: 40321245 Free PMC article. Preprint.
References
-
- Andreasen N, & Grove W (1986). Evaluation of positive and negative symptoms in schizophrenia. Psychiatry and Psychobiology, 1, 108–121.
-
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, . . . Corlett PR (2012). NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A, 109(41), 16720–16725. doi:10.1073/pnas.1208494109 - DOI - PMC - PubMed
-
- Anticevic A, & Murray JD (2017). Computational Psychiatry, Mathematical Modeling of Mental Illness, 1st edition. Academic Press.

