Integrative Genomics Analysis Identifies ACVR1B as a Candidate Causal Gene of Emphysema Distribution
- PMID: 30335480
- PMCID: PMC6444627
- DOI: 10.1165/rcmb.2018-0110OC
Integrative Genomics Analysis Identifies ACVR1B as a Candidate Causal Gene of Emphysema Distribution
Abstract
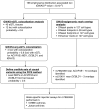
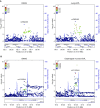
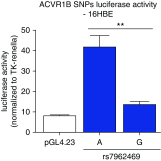
Genome-wide association studies (GWAS) have identified multiple associations with emphysema apicobasal distribution (EABD), but the biological functions of these variants are unknown. To characterize the functions of EABD-associated variants, we integrated GWAS results with 1) expression quantitative trait loci (eQTL) from the Genotype Tissue Expression (GTEx) project and subjects in the COPDGene (Genetic Epidemiology of COPD) study and 2) cell type epigenomic marks from the Roadmap Epigenomics project. On the basis of these analyses, we selected a variant near ACVR1B (activin A receptor type 1B) for functional validation. SNPs from 168 loci with P values less than 5 × 10-5 in the largest GWAS meta-analysis of EABD were analyzed. Eighty-four loci overlapped eQTL, with 12 of these loci showing greater than 80% likelihood of harboring a single, shared GWAS and eQTL causal variant. Seventeen cell types were enriched for overlap between EABD loci and Roadmap Epigenomics marks (permutation P < 0.05), with the strongest enrichment observed in CD4+, CD8+, and regulatory T cells. We selected a putative causal variant, rs7962469, associated with ACVR1B expression in lung tissue for additional functional investigation, and reporter assays confirmed allele-specific regulatory activity for this variant in human bronchial epithelial and Jurkat immune cell lines. ACVR1B expression levels exhibit a nominally significant association with emphysema distribution. EABD-associated loci are preferentially enriched in regulatory elements of multiple cell types, most notably T-cell subsets. Multiple EABD loci colocalize to regulatory elements that are active across multiple tissues and cell types, and functional analyses confirm the presence of an EABD-associated functional variant that regulates ACVR1B expression, indicating that transforming growth factor-β signaling plays a role in the EABD phenotype. Clinical trial registered with www.clinicaltrials.gov (NCT00608764).
Trial registration: ClinicalTrials.gov NCT00608764 NCT00292552.
Keywords: gene; chronic obstructive pulmonary disease; emphysema distribution; integrative genomics; transforming growth factor-β signaling.
Figures


Comment in
-
Identifying Chronic Obstructive Pulmonary Disease Genes: Shining the Light on Dark DNA.Am J Respir Cell Mol Biol. 2019 Apr;60(4):373-374. doi: 10.1165/rcmb.2018-0349ED. Am J Respir Cell Mol Biol. 2019. PMID: 30376354 Free PMC article. No abstract available.
References
-
- Celli BR. Roger S. Mitchell lecture. Chronic obstructive pulmonary disease phenotypes and their clinical relevance. Proc Am Thorac Soc. 2006;3:461–465. - PubMed
-
- de Torres JP, Bastarrika G, Zagaceta J, Sáiz-Mendiguren R, Alcaide AB, Seijo LM, et al. Emphysema presence, severity, and distribution has little impact on the clinical presentation of a cohort of patients with mild to moderate COPD. Chest. 2011;139:36–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HL113264/HL/NHLBI NIH HHS/United States
- R01 HL089897/HL/NHLBI NIH HHS/United States
- R25 ES011080/ES/NIEHS NIH HHS/United States
- R01 HL130512/HL/NHLBI NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01 HL089856/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- K08 HL141601/HL/NHLBI NIH HHS/United States
- R01 HL126596/HL/NHLBI NIH HHS/United States
- R01 HL124233/HL/NHLBI NIH HHS/United States
- R01 HL125583/HL/NHLBI NIH HHS/United States
- P01 HL132825/HL/NHLBI NIH HHS/United States
- R01 HL127200/HL/NHLBI NIH HHS/United States
- P01 HL114501/HL/NHLBI NIH HHS/United States
- T32 HL007427/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

