Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration
- PMID: 30335591
- PMCID: PMC6526812
- DOI: 10.1080/15548627.2018.1535292
Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration
Abstract
Macroautophagy/autophagy, a defense mechanism against aberrant stresses, in neurons counteracts aggregate-prone misfolded protein toxicity. Autophagy induction might be beneficial in neurodegenerative diseases (NDs). The natural compound trehalose promotes autophagy via TFEB (transcription factor EB), ameliorating disease phenotype in multiple ND models, but its mechanism is still obscure. We demonstrated that trehalose regulates autophagy by inducing rapid and transient lysosomal enlargement and membrane permeabilization (LMP). This effect correlated with the calcium-dependent phosphatase PPP3/calcineurin activation, TFEB dephosphorylation and nuclear translocation. Trehalose upregulated genes for the TFEB target and regulator Ppargc1a, lysosomal hydrolases and membrane proteins (Ctsb, Gla, Lamp2a, Mcoln1, Tpp1) and several autophagy-related components (Becn1, Atg10, Atg12, Sqstm1/p62, Map1lc3b, Hspb8 and Bag3) mostly in a PPP3- and TFEB-dependent manner. TFEB silencing counteracted the trehalose pro-degradative activity on misfolded protein causative of motoneuron diseases. Similar effects were exerted by trehalase-resistant trehalose analogs, melibiose and lactulose. Thus, limited lysosomal damage might induce autophagy, perhaps as a compensatory mechanism, a process that is beneficial to counteract neurodegeneration. Abbreviations: ALS: amyotrophic lateral sclerosis; AR: androgen receptor; ATG: autophagy related; AV: autophagic vacuole; BAG3: BCL2-associated athanogene 3; BECN1: beclin 1, autophagy related; CASA: chaperone-assisted selective autophagy; CTSB: cathepsin b; DAPI: 4',6-diamidino-2-phenylindole; DMEM: Dulbecco's modified Eagle's medium; EGFP: enhanced green fluorescent protein; fALS, familial amyotrophic lateral sclerosis; FRA: filter retardation assay; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GLA: galactosidase, alpha; HD: Huntington disease; hIPSCs: human induced pluripotent stem cells; HSPA8: heat shock protein A8; HSPB8: heat shock protein B8; IF: immunofluorescence analysis; LAMP1: lysosomal-associated membrane protein 1; LAMP2A: lysosomal-associated membrane protein 2A; LGALS3: lectin, galactose binding, soluble 3; LLOMe: L-leucyl-L-leucine methyl ester; LMP: lysosomal membrane permeabilization; Lys: lysosomes; MAP1LC3B: microtubule-associated protein 1 light chain 3 beta; MCOLN1: mucolipin 1; mRNA: messenger RNA; MTOR: mechanistic target of rapamycin kinase; NDs: neurodegenerative diseases; NSC34: neuroblastoma x spinal cord 34; PBS: phosphate-buffered saline; PD: Parkinson disease; polyQ: polyglutamine; PPARGC1A: peroxisome proliferative activated receptor, gamma, coactivator 1 alpha; PPP3CB: protein phosphatase 3, catalytic subunit, beta isoform; RT-qPCR: real-time quantitative polymerase chain reaction; SBMA: spinal and bulbar muscular atrophy; SCAs: spinocerebellar ataxias; siRNA: small interfering RNA; SLC2A8: solute carrier family 2, (facilitated glucose transporter), member 8; smNPCs: small molecules neural progenitors cells; SOD1: superoxide dismutase 1; SQSTM1/p62: sequestosome 1; STED: stimulated emission depletion; STUB1: STIP1 homology and U-box containing protein 1; TARDBP/TDP-43: TAR DNA binding protein; TFEB: transcription factor EB; TPP1: tripeptidyl peptidase I; TREH: trehalase (brush-border membrane glycoprotein); WB: western blotting; ZKSCAN3: zinc finger with KRAB and SCAN domains 3.
Keywords: Amyotrophic lateral sclerosis; TFEB; autophagy; calcineurin; galectin-3; lactulose; lysosomes; melibiose; motoneuron diseases; neurodegeneration; protein quality control; spinal and bulbar muscular atrophy; trehalose.
Figures
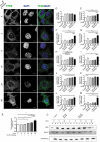
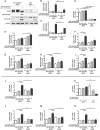

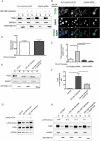
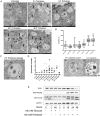
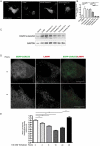
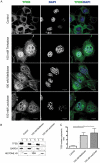
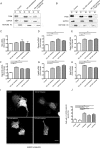

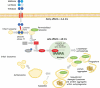
References
-
- Lumkwana D, Du Toit A, Kinnear C, et al. Autophagic flux control in neurodegeneration: progress and precision targeting-Where do we stand? Progr Neurobiol. 2017;153:64–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
