Preservation of myocardial contractility during acute hypoxia with OMX-CV, a novel oxygen delivery biotherapeutic
- PMID: 30335746
- PMCID: PMC6193608
- DOI: 10.1371/journal.pbio.2005924
Preservation of myocardial contractility during acute hypoxia with OMX-CV, a novel oxygen delivery biotherapeutic
Erratum in
-
Correction: Preservation of myocardial contractility during acute hypoxia with OMX-CV, a novel oxygen delivery biotherapeutic.PLoS Biol. 2019 Jan 24;17(1):e3000119. doi: 10.1371/journal.pbio.3000119. eCollection 2019 Jan. PLoS Biol. 2019. PMID: 30677022 Free PMC article.
Abstract
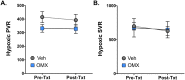
The heart exhibits the highest basal oxygen (O2) consumption per tissue mass of any organ in the body and is uniquely dependent on aerobic metabolism to sustain contractile function. During acute hypoxic states, the body responds with a compensatory increase in cardiac output that further increases myocardial O2 demand, predisposing the heart to ischemic stress and myocardial dysfunction. Here, we test the utility of a novel engineered protein derived from the heme-based nitric oxide (NO)/oxygen (H-NOX) family of bacterial proteins as an O2 delivery biotherapeutic (Omniox-cardiovascular [OMX-CV]) for the hypoxic myocardium. Because of their unique binding characteristics, H-NOX-based variants effectively deliver O2 to hypoxic tissues, but not those at physiologic O2 tension. Additionally, H-NOX-based variants exhibit tunable binding that is specific for O2 with subphysiologic reactivity towards NO, circumventing a significant toxicity exhibited by hemoglobin (Hb)-based O2 carriers (HBOCs). Juvenile lambs were sedated, mechanically ventilated, and instrumented to measure cardiovascular parameters. Biventricular admittance catheters were inserted to perform pressure-volume (PV) analyses. Systemic hypoxia was induced by ventilation with 10% O2. Following 15 minutes of hypoxia, the lambs were treated with OMX-CV (200 mg/kg IV) or vehicle. Acute hypoxia induced significant increases in heart rate (HR), pulmonary blood flow (PBF), and pulmonary vascular resistance (PVR) (p < 0.05). At 1 hour, vehicle-treated lambs exhibited severe hypoxia and a significant decrease in biventricular contractile function. However, in OMX-CV-treated animals, myocardial oxygenation was improved without negatively impacting systemic or PVR, and both right ventricle (RV) and left ventricle (LV) contractile function were maintained at pre-hypoxic baseline levels. These data suggest that OMX-CV is a promising and safe O2 delivery biotherapeutic for the preservation of myocardial contractility in the setting of acute hypoxia.
Conflict of interest statement
EM and JRF are consultants to Omniox, Inc.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

