Imaging breast cancer using a dual-ligand nanochain particle
- PMID: 30335750
- PMCID: PMC6193613
- DOI: 10.1371/journal.pone.0204296
Imaging breast cancer using a dual-ligand nanochain particle
Abstract
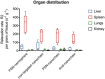
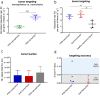
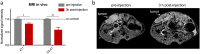
Nanoparticles often only exploit the upregulation of a receptor on cancer cells to enhance intratumoral deposition of therapeutic and imaging agents. However, a single targeting moiety assumes that a tumor is homogenous and static. Tumoral microenvironments are both heterogenous and dynamic, often displaying variable spatial and temporal expression of targetable receptors throughout disease progression. Here, we evaluated the in vivo performance of an iron oxide nanoparticle in terms of targeting and imaging of orthotropic mouse models of aggressive breast tumors. The nanoparticle, a multi-component nanochain, was comprised of 3-5 iron oxide nanoparticles chemically linked in a linear chain. The nanoparticle's surface was decorated with two types of ligands each targeting two different upregulated biomarkers on the tumor endothelium, P-selectin and fibronectin. The nanochain exhibited improved tumor deposition not only through vascular targeting but also through its elongated structure. A single-ligand nanochain exhibited a ~2.5-fold higher intratumoral deposition than a spherical nanoparticle variant. Furthermore, the dual-ligand nanochain exhibited higher consistency in generating detectable MR signals compared to a single-ligand nanochain. Using a 7T MRI, the dual-ligand nanochains exhibited highly detectable MR signal within 3h after injection in two different animal models of breast cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

