Identification of UHRF2 as a novel DNA interstrand crosslink sensor protein
- PMID: 30335751
- PMCID: PMC6193622
- DOI: 10.1371/journal.pgen.1007643
Identification of UHRF2 as a novel DNA interstrand crosslink sensor protein
Abstract
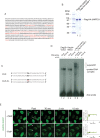
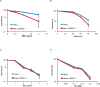
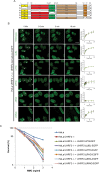
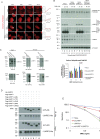
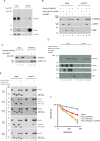
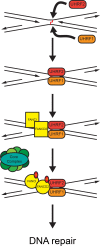
The Fanconi Anemia (FA) pathway is important for repairing interstrand crosslinks (ICLs) between the Watson-Crick strands of the DNA double helix. An initial and essential stage in the repair process is the detection of the ICL. Here, we report the identification of UHRF2, a paralogue of UHRF1, as an ICL sensor protein. UHRF2 is recruited to ICLs in the genome within seconds of their appearance. We show that UHRF2 cooperates with UHRF1, to ensure recruitment of FANCD2 to ICLs. A direct protein-protein interaction is formed between UHRF1 and UHRF2, and between either UHRF1 and UHRF2, and FANCD2. Importantly, we demonstrate that the essential monoubiquitination of FANCD2 is stimulated by UHRF1/UHRF2. The stimulation is mediating by a retention of FANCD2 on chromatin, allowing for its monoubiquitination by the FA core complex. Taken together, we uncover a mechanism of ICL sensing by UHRF2, leading to FANCD2 recruitment and retention at ICLs, in turn facilitating activation of FANCD2 by monoubiquitination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

