Uncovering vector, parasite, blood meal and microbiome patterns from mixed-DNA specimens of the Chagas disease vector Triatoma dimidiata
- PMID: 30335763
- PMCID: PMC6193617
- DOI: 10.1371/journal.pntd.0006730
Uncovering vector, parasite, blood meal and microbiome patterns from mixed-DNA specimens of the Chagas disease vector Triatoma dimidiata
Abstract
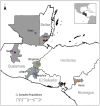
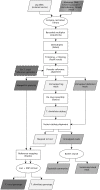
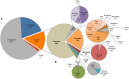
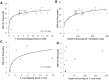
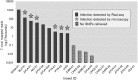
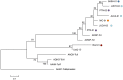
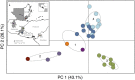
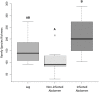
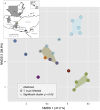
Chagas disease, considered a neglected disease by the World Health Organization, is caused by the protozoan parasite Trypanosoma cruzi, and transmitted by >140 triatomine species across the Americas. In Central America, the main vector is Triatoma dimidiata, an opportunistic blood meal feeder inhabiting both domestic and sylvatic ecotopes. Given the diversity of interacting biological agents involved in the epidemiology of Chagas disease, having simultaneous information on the dynamics of the parasite, vector, the gut microbiome of the vector, and the blood meal source would facilitate identifying key biotic factors associated with the risk of T. cruzi transmission. In this study, we developed a RADseq-based analysis pipeline to study mixed-species DNA extracted from T. dimidiata abdomens. To evaluate the efficacy of the method across spatial scales, we used a nested spatial sampling design that spanned from individual villages within Guatemala to major biogeographic regions of Central America. Information from each biotic source was distinguished with bioinformatics tools and used to evaluate the prevalence of T. cruzi infection and predominant Discrete Typing Units (DTUs) in the region, the population genetic structure of T. dimidiata, gut microbial diversity, and the blood meal history. An average of 3.25 million reads per specimen were obtained, with approximately 1% assigned to the parasite, 20% to the vector, 11% to bacteria, and 4% to putative blood meals. Using a total of 6,405 T. cruzi SNPs, we detected nine infected vectors harboring two distinct DTUs: TcI and a second unidentified strain, possibly TcIV. Vector specimens were sufficiently variable for population genomic analyses, with a total of 25,710 T. dimidiata SNPs across all samples that were sufficient to detect geographic genetic structure at both local and regional scales. We observed a diverse microbiotic community, with significantly higher bacterial species richness in infected T. dimidiata abdomens than those that were not infected. Unifrac analysis suggests a common assemblage of bacteria associated with infection, which co-occurs with the typical gut microbial community derived from the local environment. We identified vertebrate blood meals from five T. dimidiata abdomens, including chicken, dog, duck and human; however, additional detection methods would be necessary to confidently identify blood meal sources from most specimens. Overall, our study shows this method is effective for simultaneously generating genetic data on vectors and their associated parasites, along with ecological information on feeding patterns and microbial interactions that may be followed up with complementary approaches such as PCR-based parasite detection, 18S eukaryotic and 16S bacterial barcoding.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures
References
-
- World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015. February; 90(6): 33–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

