cGMP production of astatine-211-labeled anti-CD45 antibodies for use in allogeneic hematopoietic cell transplantation for treatment of advanced hematopoietic malignancies
- PMID: 30335787
- PMCID: PMC6193629
- DOI: 10.1371/journal.pone.0205135
cGMP production of astatine-211-labeled anti-CD45 antibodies for use in allogeneic hematopoietic cell transplantation for treatment of advanced hematopoietic malignancies
Abstract
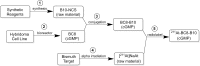
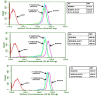
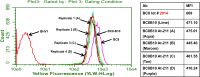
The objective of this study was to translate reaction conditions and quality control methods used for production of an astatine-211(211At)-labeled anti-CD45 monoclonal antibody (MAb) conjugate, 211At-BC8-B10, from the laboratory setting to cGMP production. Five separate materials were produced in the preparation of 211At-BC8-B10: (1) p-isothiocyanato-phenethyl-closo-decaborate(2-) (B10-NCS), (2) anti-CD45 MAb, BC8, (3) BC8-B10 MAb conjugate, (4) [211At]NaAt, and (5) 211At-BC8-B10. The 211At-labeling reagent, B10-NCS, was synthesized as previously reported. BC8 was produced, then conjugated with B10-NCS under cGMP conditions to form BC8-B10. [211At]NaAt was produced by α-irradiation of Bi targets, followed by isolation of the 211At using a "wet chemistry" method. The clinical product, 211At-BC8-B10, was prepared by reacting [211At]NaAt with BC8-B10 in NH4OAc buffer (pH 5.5) for 2 min at room temperature, followed by size-exclusion chromatography purification. Quality control tests conducted on the 211At-BC8-B10 included evaluations for purity and identity, as well as pyrogen and sterility tests. Stability of the 211At-BC8-B10 in 25 mg/mL sodium ascorbate solution was evaluated at 1, 2, 4, 6 and 21 h post isolation. For qualification, three consecutive 211At-BC8-B10 clinical preparations were successfully conducted in the cGMP suite, and an additional cGMP clinical preparation was carried out to validate each step required to deliver 211At-BC8-B10 to a patient. These cGMP preparations provided 0.80-1.28 Gbq (21.5-34.5 mCi) of 211At-BC8-B10 with radiochemical purity of >97%. The preparations were found to be sterile and have a pyrogen level <0.50 EU/mL. Cell binding was retained by the 211At-BC8-B10. 211At-BC8-B10 in ascorbic acid solution demonstrated a radiochemical stability of >95% for up to 21 h at room temperature. The experiments conducted have defined conditions for translation of 211At-BC8-B10 production from the laboratory to cGMP suite. This study has allowed the initiation of a phase I/II clinical trial using 211At-BC8-B10 (NCT03128034).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

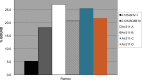
References
-
- Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Bush SA, et al. Development of a Marrow Transplant Regimen for Acute Leukemia Using Targeted Hematopoietic Irradiation Delivered by 131I-Labeled Anti-CD45 Antibody, Combined With Cyclophosphamide and Total Body Irradiation. Blood. 1995;85:1122–31. - PubMed
-
- Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Hui TE, et al. Phase I study of 131I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94(4):1237–47. - PubMed
-
- Press OW, Eary JF, Appelbaum FR, Martin PJ, Nelp WB, Glenn S, et al. Phase II trial of 131I-B1 (anti-CD20) antibody therapy with autologous stem cell transplantation for relapsed B cell lymphomas. Lancet. 1995;346:336–40. - PubMed
-
- Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107(5):2184–91. Epub 2005/10/29. doi: 2005-06-2317 [pii] 10.1182/blood-2005-06-2317 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

