Implications of the expression of Enterococcus faecalis citrate fermentation genes during infection
- PMID: 30335810
- PMCID: PMC6193673
- DOI: 10.1371/journal.pone.0205787
Implications of the expression of Enterococcus faecalis citrate fermentation genes during infection
Abstract
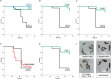
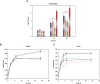
Citrate is an ubiquitous compound in nature. However, citrate fermentation is present only in a few pathogenic or nonpathogenic microorganisms. The citrate fermentation pathway includes a citrate transporter, a citrate lyase complex, an oxaloacetate decarboxylase and a regulatory system. Enterococcus faecalis is commonly present in the gastro-intestinal microbiota of warm-blooded animals and insect guts. These bacteria can also cause infection and disease in immunocompromised individuals. In the present study, we performed whole genome analysis in Enterococcus strains finding that the complete citrate pathway is present in all of the E. faecalis strains isolated from such diverse habitats as animals, hospitals, water, milk, plants, insects, cheese, etc. These results indicate the importance of this metabolic preservation for persistence and growth of E. faecalis in different niches. We also analyzed the role of citrate metabolism in the E. faecalis pathogenicity. We found that an E. faecalis citrate fermentation-deficient strain was less pathogenic for Galleria mellonella larvae than the wild type. Furthermore, strains with deletions in the oxaloacetate decarboxylase subunits or in the α-acetolactate synthase resulted also less virulent than the wild type strain. We also observed that citrate promoters are induced in blood, urine and also in the hemolymph of G. mellonella. In addition, we showed that citrate fermentation allows E. faecalis to grow better in blood, urine and G. mellonella. The results presented here clearly indicate that citrate fermentation plays an important role in E. faecalis opportunistic pathogenic behavior.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Transcriptional regulation of the citrate gene cluster of Enterococcus faecalis Involves the GntR family transcriptional activator CitO.J Bacteriol. 2008 Nov;190(22):7419-30. doi: 10.1128/JB.01704-07. Epub 2008 Sep 19. J Bacteriol. 2008. PMID: 18805984 Free PMC article.
-
Identification of malic and soluble oxaloacetate decarboxylase enzymes in Enterococcus faecalis.FEBS J. 2011 Jun;278(12):2140-51. doi: 10.1111/j.1742-4658.2011.08131.x. Epub 2011 May 17. FEBS J. 2011. PMID: 21518252
-
Aroma compounds generation in citrate metabolism of Enterococcus faecium: Genetic characterization of type I citrate gene cluster.Int J Food Microbiol. 2016 Feb 2;218:27-37. doi: 10.1016/j.ijfoodmicro.2015.11.004. Epub 2015 Nov 14. Int J Food Microbiol. 2016. PMID: 26594791
-
Molecular Mechanism of Quorum-Sensing in Enterococcus faecalis: Its Role in Virulence and Therapeutic Approaches.Int J Mol Sci. 2017 May 3;18(5):960. doi: 10.3390/ijms18050960. Int J Mol Sci. 2017. PMID: 28467378 Free PMC article. Review.
-
Lipoproteins of Enterococcus faecalis: bioinformatic identification, expression analysis and relation to virulence.Microbiology (Reading). 2011 Nov;157(Pt 11):3001-3013. doi: 10.1099/mic.0.053314-0. Epub 2011 Sep 8. Microbiology (Reading). 2011. PMID: 21903750 Review.
Cited by
-
Genome and transcriptome analysis of Enterococcus faecium from intestinal colonization and Enterococcus faecium from urinary tract infection.Front Microbiol. 2023 Oct 31;14:1273949. doi: 10.3389/fmicb.2023.1273949. eCollection 2023. Front Microbiol. 2023. PMID: 38029192 Free PMC article.
-
Changes of gut microbiota and tricarboxylic acid metabolites may be helpful in early diagnosis of necrotizing enterocolitis: A pilot study.Front Microbiol. 2023 Mar 15;14:1119981. doi: 10.3389/fmicb.2023.1119981. eCollection 2023. Front Microbiol. 2023. PMID: 37007499 Free PMC article.
-
Enhancing colistin efficacy against Salmonella infections with a quinazoline-based dual therapeutic strategy.Sci Rep. 2024 Mar 1;14(1):5148. doi: 10.1038/s41598-024-55793-0. Sci Rep. 2024. PMID: 38429351 Free PMC article.
-
Over supplementation with vitamin B12 alters microbe-host interactions in the gut leading to accelerated Citrobacter rodentium colonization and pathogenesis in mice.Microbiome. 2023 Feb 3;11(1):21. doi: 10.1186/s40168-023-01461-w. Microbiome. 2023. PMID: 36737826 Free PMC article.
-
Redundant potassium transporter systems guarantee the survival of Enterococcus faecalis under stress conditions.Front Microbiol. 2023 Feb 8;14:1117684. doi: 10.3389/fmicb.2023.1117684. eCollection 2023. Front Microbiol. 2023. PMID: 36846772 Free PMC article.
References
-
- Zuljan FA, Mortera P, Alarcón SH, Blancato VS, Espariz M, Magni C. Lactic acid bacteria decarboxylation reactions in cheese. International Dairy Journal. 2016;62:53–62. 10.1016/j.idairyj.2016.07.007 - DOI
-
- Vebo HC, Solheim M, Snipen L, Nes IF, Brede DA. Comparative genomic analysis of pathogenic and probiotic Enterococcus faecalis isolates, and their transcriptional responses to growth in human urine. PloS one. 2010;5(8):e12489 10.1371/journal.pone.0012489 ; PubMed Central PMCID: PMC2930860. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

