Independent amplification of co-infected long incubation period low conversion efficiency prion strains
- PMID: 30335854
- PMCID: PMC6193734
- DOI: 10.1371/journal.ppat.1007323
Independent amplification of co-infected long incubation period low conversion efficiency prion strains
Abstract
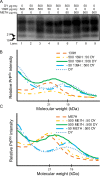
Prion diseases are caused by a misfolded isoform of the prion protein, PrPSc. Prion strains are hypothesized to be encoded by strain-specific conformations of PrPSc and prions can interfere with each other when a long-incubation period strain (i.e. blocking strain) inhibits the conversion of a short-incubation period strain (i.e. non-blocking). Prion strain interference influences prion strain dynamics and the emergence of a strain from a mixture; however, it is unknown if two long-incubation period strains can interfere with each other. Here, we show that co-infection of animals with combinations of long-incubation period strains failed to identify evidence of strain interference. To exclude the possibility that this inability of strains to interfere in vivo was due to a failure to infect common populations of neurons we used protein misfolding cyclic amplification strain interference (PMCAsi). Consistent with the animal bioassay studies, PMCAsi indicated that both co-infecting strains were amplifying independently, suggesting that the lack of strain interference is not due to a failure to target the same cells but is an inherent property of the strains involved. Importantly PMCA reactions seeded with long incubation-period strains contained relatively higher levels of remaining PrPC compared to reactions seeded with a short-incubation period strain. Mechanistically, we hypothesize the abundance of PrPC is not limiting in vivo or in vitro resulting in prion strains with relatively low prion conversion efficiency to amplify independently. Overall, this observation changes the paradigm of the interactions of prion strains and has implications for interspecies transmission and emergence of prion strains from a mixture.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Incongruity between Prion Conversion and Incubation Period following Coinfection.J Virol. 2016 May 27;90(12):5715-23. doi: 10.1128/JVI.00409-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27053546 Free PMC article.
-
Prion formation, but not clearance, is supported by protein misfolding cyclic amplification.Prion. 2014;8(6):415-20. doi: 10.4161/19336896.2014.983759. Prion. 2014. PMID: 25482601 Free PMC article.
-
Nonpathogenic Heterologous Prions Can Interfere with Prion Infection in a Strain-Dependent Manner.J Virol. 2018 Nov 27;92(24):e01086-18. doi: 10.1128/JVI.01086-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282706 Free PMC article.
-
Prion protein conversion in vitro.J Mol Med (Berl). 2004 Jun;82(6):348-56. doi: 10.1007/s00109-004-0534-3. Epub 2004 Mar 10. J Mol Med (Berl). 2004. PMID: 15014886 Review.
-
Prion protein aggregation and fibrillogenesis in vitro.Subcell Biochem. 2012;65:91-108. doi: 10.1007/978-94-007-5416-4_5. Subcell Biochem. 2012. PMID: 23225001 Review.
Cited by
-
Multiple aspects of amyloid dynamics in vivo integrate to establish prion variant dominance in yeast.Front Mol Neurosci. 2024 Jul 30;17:1439442. doi: 10.3389/fnmol.2024.1439442. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39139213 Free PMC article.
-
In vitro detection of haematogenous prions in white-tailed deer orally dosed with low concentrations of chronic wasting disease.J Gen Virol. 2020 Mar;101(3):347-361. doi: 10.1099/jgv.0.001367. J Gen Virol. 2020. PMID: 31846418 Free PMC article.
-
BSE can propagate in sheep co-infected or pre-infected with scrapie.Sci Rep. 2021 Jun 7;11(1):11931. doi: 10.1038/s41598-021-91397-8. Sci Rep. 2021. PMID: 34099797 Free PMC article.
-
Prion strains: shining new light on old concepts.Cell Tissue Res. 2023 Apr;392(1):113-133. doi: 10.1007/s00441-022-03665-2. Epub 2022 Jul 7. Cell Tissue Res. 2023. PMID: 35796874 Free PMC article. Review.
-
Effect of host and strain factors on α-synuclein prion pathogenesis.Trends Neurosci. 2024 Jul;47(7):538-550. doi: 10.1016/j.tins.2024.05.004. Epub 2024 May 27. Trends Neurosci. 2024. PMID: 38806297 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

