Fungal persister cells: The basis for recalcitrant infections?
- PMID: 30335865
- PMCID: PMC6193731
- DOI: 10.1371/journal.ppat.1007301
Fungal persister cells: The basis for recalcitrant infections?
Abstract
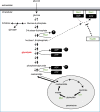
Persister cells are a small subpopulation within fungal biofilms that are highly resistant to high concentrations of antifungals and therefore most likely contribute to the resistance and recalcitrance of biofilm infections. Moreover, this subpopulation is defined as a nongrowing, phenotypic variant of wild-type cells that can survive high doses of antifungals. There are high degrees of heterogeneity and plasticity associated with biofilm formation, resulting in a strong variation in the amount of persister cells. The fraction of these cells in fungal biofilms also appear to be dependent on the type of substrate. The cells can be observed immediately after their adhesion to that substrate, which makes up the initial step of biofilm formation. Thus far, persister cells have primarily been studied in Candida spp. These fungi are the fourth most common cause of nosocomial systemic infections in the United States, with C. albicans being the most prevalent species. Remarkably, persisters exhibit characteristics of a dormant state similar to what is observed in cells deprived of glucose. This dormant state, together with attachment to a substrate, appears to provide the cells with characteristics that help them overcome the challenges with fungicidal drugs such as amphotericin B (AmB). AmB is known to induce apoptosis, and persister cells are able to cope with the increase in reactive oxygen species (ROS) by activating stress response pathways and the accumulation of high amounts of glycogen and trehalose-two known stress-protecting molecules. In this review, we discuss the molecular pathways that are involved in persister cell formation in fungal species and highlight that the eradication of persister cells could lead to a strong reduction of treatment failure in a clinical setting.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Andes DR, Safdar N, Baddley JW, Playford G, Reboli AC, Rex JH, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54: 1110–1122. 10.1093/cid/cis021 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

