Enzymatic Transition States and Drug Design
- PMID: 30335982
- PMCID: PMC6615489
- DOI: 10.1021/acs.chemrev.8b00369
Enzymatic Transition States and Drug Design
Abstract
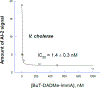
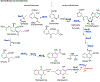
Transition state theory teaches that chemically stable mimics of enzymatic transition states will bind tightly to their cognate enzymes. Kinetic isotope effects combined with computational quantum chemistry provides enzymatic transition state information with sufficient fidelity to design transition state analogues. Examples are selected from various stages of drug development to demonstrate the application of transition state theory, inhibitor design, physicochemical characterization of transition state analogues, and their progress in drug development.
Conflict of interest statement
The author declares the following competing financial interest(s): The author serves as a consultant or board member to several biotech companies involved in development of the immucillin family of transition state analogues, some of them described here. The intellectual property developed around these inhibitors is the joint property of the Albert Einstein College of Medicine and Victoria University of Wellington, New Zealand. The author and collaborators receive royalties through dividend plans of these institutions.
Figures
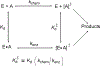


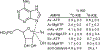

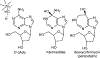
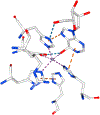







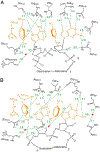

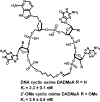
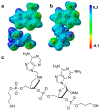
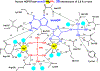






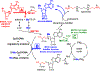
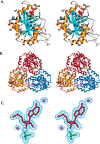
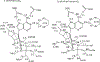
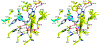

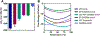
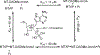
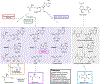
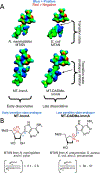

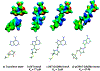








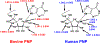

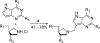
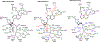

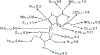

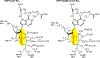






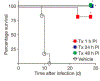

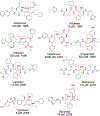
Similar articles
-
Enzymatic transition states: thermodynamics, dynamics and analogue design.Arch Biochem Biophys. 2005 Jan 1;433(1):13-26. doi: 10.1016/j.abb.2004.08.035. Arch Biochem Biophys. 2005. PMID: 15581562 Review.
-
Transition States, analogues, and drug development.ACS Chem Biol. 2013 Jan 18;8(1):71-81. doi: 10.1021/cb300631k. Epub 2013 Jan 4. ACS Chem Biol. 2013. PMID: 23259601 Free PMC article. Review.
-
Enzymatic transition states and transition state analog design.Annu Rev Biochem. 1998;67:693-720. doi: 10.1146/annurev.biochem.67.1.693. Annu Rev Biochem. 1998. PMID: 9759501 Review.
-
Enzymatic transition states, transition-state analogs, dynamics, thermodynamics, and lifetimes.Annu Rev Biochem. 2011;80:703-32. doi: 10.1146/annurev-biochem-061809-100742. Annu Rev Biochem. 2011. PMID: 21675920 Free PMC article. Review.
-
Enzyme inhibitors as chemical tools to study enzyme catalysis: rational design, synthesis, and applications.Chem Rec. 2005;5(4):209-28. doi: 10.1002/tcr.20045. Chem Rec. 2005. PMID: 16041744 Review.
Cited by
-
Matching Glycosyl Donor Reactivity to Sulfonate Leaving Group Ability Permits SN2 Glycosylations.J Am Chem Soc. 2019 Oct 23;141(42):16743-16754. doi: 10.1021/jacs.9b07022. Epub 2019 Oct 9. J Am Chem Soc. 2019. PMID: 31550879 Free PMC article.
-
MAT Gain of Activity Mutation in Helicobacter pylori Is Associated with Resistance to MTAN Transition State Analogues.ACS Infect Dis. 2023 Apr 14;9(4):966-978. doi: 10.1021/acsinfecdis.2c00644. Epub 2023 Mar 15. ACS Infect Dis. 2023. PMID: 36920074 Free PMC article.
-
Antiviral and Antimicrobial Nucleoside Derivatives: Structural Features and Mechanisms of Action.Mol Biol. 2021;55(6):786-812. doi: 10.1134/S0026893321040105. Epub 2021 Dec 17. Mol Biol. 2021. PMID: 34955556 Free PMC article.
-
Enzyme dynamics: Looking beyond a single structure.ChemCatChem. 2020 Oct 6;12(19):4704-4720. doi: 10.1002/cctc.202000665. Epub 2020 Jun 26. ChemCatChem. 2020. PMID: 33897908 Free PMC article.
-
Neutron structures of Leishmania mexicana triosephosphate isomerase in complex with reaction-intermediate mimics shed light on the proton-shuttling steps.IUCrJ. 2021 Jun 3;8(Pt 4):633-643. doi: 10.1107/S2052252521004619. eCollection 2021 Jul 1. IUCrJ. 2021. PMID: 34258011 Free PMC article.
References
-
- Pauling L Molecular Architecture and Biological Reactions. Chem. Eng. News 1946, 24, 1375–1377.
-
- Pauling L Nature of Forces Between Large Molecules of Biological Interest. Nature 1948, 161, 707–709. - PubMed
-
- Wolfenden R Transition State Analogues for Enzyme Catalysis. Nature 1969, 223, 704–705. - PubMed
-
- Wolfenden R Transition State Analog Inhibitors and Enzyme Catalysis. Annu. Rev. Biophys. Bioeng 1976, 5, 271–306. - PubMed
-
- Wolfenden R Thermodynamic and Extrathermodynamic Requirements of Enzyme Catalysis. Biophys. Chem 2003, 105, 559–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

