PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake
- PMID: 30336167
- PMCID: PMC6477695
- DOI: 10.1016/j.jconrel.2018.10.015
PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake
Abstract
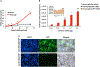
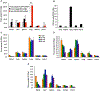
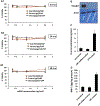
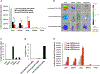
Systemic administration of lipid nanoparticle (LNP)-encapsulated messenger RNA (mRNA) leads predominantly to hepatic uptake and expression. Here, we conjugated nucleoside-modified mRNA-LNPs with antibodies (Abs) specific to vascular cell adhesion molecule, PECAM-1. Systemic (intravenous) administration of Ab/LNP-mRNAs resulted in profound inhibition of hepatic uptake concomitantly with ~200-fold and 25-fold elevation of mRNA delivery and protein expression in the lungs compared to non-targeted counterparts. Unlike hepatic delivery of LNP-mRNA, Ab/LNP-mRNA is independent of apolipoprotein E. Vascular re-targeting of mRNA represents a promising, powerful, and unique approach for novel experimental and clinical interventions in organs of interest other than liver.
Keywords: Apolipoprotein E; Endothelial targeting; Inflammation; Vascular targeting; mRNA delivery.
Copyright © 2018. Published by Elsevier B.V.
Conflict of interest statement
Conflict of interest
H.P., V.V.S., D.W., and V.R.M. are inventors on a patent filed on some aspects of this work. Those interests have been fully disclosed to the University of Pennsylvania.
Figures



References
-
- Sahin U, Kariko K, Tureci O, mRNA-based therapeutics-developing a new class of drugs, Nat. Rev. Drug Discov 13 (2014) 759–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

