AST-120 Reduces Neuroinflammation Induced by Indoxyl Sulfate in Glial Cells
- PMID: 30336612
- PMCID: PMC6210605
- DOI: 10.3390/jcm7100365
AST-120 Reduces Neuroinflammation Induced by Indoxyl Sulfate in Glial Cells
Abstract
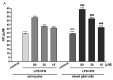
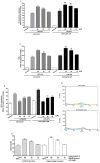
Chronic kidney disease (CKD) involves multiple organ dysfunction, and the neurological complications that are often present in CKD patients support the idea of a crosstalk between the kidneys and the brain. Evidence suggests a possible role for products accumulating in these patients as uremic toxins in various CKD complications, including neurodegeneration. Indoxyl sulfate (IS), derived from tryptophan metabolism, is well-known as a uremic nephron-vascular toxin, and recent evidence suggests it also has a role in the immune response and in neurodegeneration. Inflammation has been associated with neurodegenerative diseases, as well as with CKD. In this study, we demonstrated that sera of CKD patients induced a significant inflammation in astrocyte cells which was proportional to IS sera concentrations, and that the IS adsorbent, AST-120, reduced this inflammatory response. These results indicated that, among the uremic toxins accumulating in serum of CKD patients, IS significantly contributed to astrocyte inflammation. Moreover, being also chronic inflammation associated with CKD, here we reported that IS further increased inflammation and oxidative stress in primary central nervous system (CNS) cells, via Nuclear Factor-κB (NF-κB) and Aryl hydrocarbon Receptor (AhR) activation, and induced neuron death. This study is a step towards elucidating IS as a potential pharmacological target in CKD patients.
Keywords: AST-120; chronic kidney disease; glial cells; indoxyl sulfate; neuroinflammation.
Conflict of interest statement
Masaki Fujioka is employee of Kureha Corporation (Tokyo, Japan). He had no influence on interpretation of study results and the decision to submit the manuscript for publication. The other authors had no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources

