Synthesis of Phthalans Via Copper-Catalyzed Enantioselective Cyclization/Carboetherification of 2-Vinylbenzyl Alcohols
- PMID: 30336677
- PMCID: PMC6459690
- DOI: 10.1021/acs.orglett.8b02766
Synthesis of Phthalans Via Copper-Catalyzed Enantioselective Cyclization/Carboetherification of 2-Vinylbenzyl Alcohols
Abstract
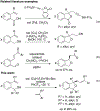
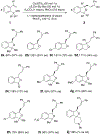
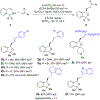
Enantiomerically enriched phthalans were synthesized efficiently via an enantioselective copper-catalyzed alkene carboetherification reaction. In this reaction, 2-vinylbenzyl alcohols enantioselectively cyclize then couple with vinylarenes. The utility of the method was demonstrated by the enantioselective synthesis of ( R)-fluspidine, a σ1 receptor ligand.
Figures
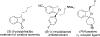


References
-
- Karmakar R; Pahari P; Mal D Chem. Rev 2014, 114, 6213–6284. - PubMed
- Maestrup EG; Wiese C; Schepmann D; Brust P; Wunsch B Bioorg. Med. Chem 2011, 19, 393–405. - PubMed
- Holl K; Falck E; Kohler J; Schepmann D; Humpf H-U; Brust P; Wunsch B ChemMedChem 2013, 8, 2047–2056. - PubMed
- Brust P; Deuther-Conrad W; Becker G; Patt M; Donat CK; Stittsworth S; Fischer S; Hiller A; Wenzel B; Dukic-Stefanovic S; Hesse S; Steinbach J; Wunsch B; Lever SZ; Sabri OJ Nucl. Med 2014, 55, 1730–1736. - PubMed
- Nakane S; Yoshinaka S; Iwase S; Shuto Y; Bunse P; Wunsch B; Tanaka S; Kitamura M Tetrahedron 2018, 74, 5069–5084.
-
- Kobayashi K; Shikata K; Fukamachi S; Konishi H Heterocycles 2008, 75, 599–609.
-
- Nicolai S; Erard S; Gonzalez DF; Waser J Org. Lett 2010, 12, 384–387. - PubMed
- Ha TM; Wang Q; Zhu J Chem. Commun 2016, 52, 11100–11103. - PubMed
- Xie J; Wang Y-W; Qi L-W; Zhang B Org. Lett 2017, 19, 1148–1151 Reviews of alkene carboetherification by oxymetallation: . - PubMed
- Wolfe JP Synlett 2008, 2008, 2913–2937.
- Garlets ZJ; White DR; Wolfe JP Asian J. Org. Chem 2017, 6, 636–653. - PMC - PubMed
- McDonald RI; Liu G; Stahl SS Chem. Rev 2011, 111, 2981–3019. - PMC - PubMed
- Chemler SR; Karyakarte SD; Khoder ZM J. Org. Chem 2017, 82, 11311–11325. - PMC - PubMed
-
- Reddy RS; Kiran INC; Sudalai A Org. Biomol. Chem 2012, 10, 3655–3661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

