A Randomized Trial Comparing Extended-Release Injectable Suspension and Oral Naltrexone, Both Combined With Behavioral Therapy, for the Treatment of Opioid Use Disorder
- PMID: 30336703
- PMCID: PMC6358483
- DOI: 10.1176/appi.ajp.2018.17070732
A Randomized Trial Comparing Extended-Release Injectable Suspension and Oral Naltrexone, Both Combined With Behavioral Therapy, for the Treatment of Opioid Use Disorder
Abstract
Objective: The oral formulation of the opioid antagonist naltrexone has shown limited effectiveness for treatment of opioid use disorder due to poor adherence. Long-acting injection naltrexone (XR-naltrexone), administered monthly, circumvents the need for daily pill taking, potentially improving adherence, and has been shown to be superior to placebo in reducing opioid use over 6 months of treatment. This open-label trial compared the outcomes of patients with opioid use disorder treated with XR-naltrexone or oral naltrexone in combination with behavioral therapy.
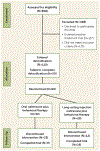
Method: Sixty opioid-dependent adults completed inpatient opioid withdrawal and were transitioned to oral naltrexone. They were stratified by severity of opioid use (six or fewer bags versus more than six bags of heroin per day) and randomly assigned (1:1) to continue treatment with oral naltrexone (N=32) or XR-naltrexone (N=28) for 24 weeks. The first dose of XR-naltrexone (380 mg) was administered prior to discharge, with monthly doses thereafter, and oral naltrexone was given in a 50-mg daily dose. All participants received weekly behavioral therapy to support treatment and adherence to naltrexone.
Results: A Cox proportional hazards model adjusting for race, gender, route of use, and baseline opioid use severity indicated that significantly more patients were retained in treatment for 6 months in the XR-naltrexone group (16 of 28 patients, 57.1%) than in the oral naltrexone group (nine of 32 patients, 28.1%) (hazard ratio=2.18, 95% CI=1.07, 4.43).
Conclusions: Patients receiving XR-naltrexone had twice the rate of treatment retention at 6 months compared with those taking oral naltrexone. These results support the use of XR-naltrexone combined with behavioral therapy as an effective treatment for patients seeking opioid withdrawal and nonagonist treatment for preventing relapse to opioid use disorder.
Keywords: Injection Naltrexone; Opioid Antagonist; Opioid Use Disorder; Oral Naltrexone; Treatment Retention.
Figures

References
-
- Centers for Disease Control and Prevention: Increases in Drug and Opioid-Involved Overdose Deaths: United States, 2010–2015. Morbidity and Mortality Weekly Report (MMWR) 65(50–51):1445–1452. https://www.cdc.gov/mmwr/volumes/65/wr/mm655051e1.htm - PubMed
-
- Johansson BA, Berglund M, Lindgren A: Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction 2006; 101:491–503 - PubMed
-
- Brewer C: Combining pharmacological antagonists and behavioural psychotherapy in treating addictions. Why it is effective but unpopular. Br J Psychiatry 1990; 157:34–40 - PubMed
-
- Preston KL, Silverman K, Umbricht A, et al.: Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend 1999; 54:127–135 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

