DAXX promotes ovarian cancer ascites cell proliferation and migration by activating the ERK signaling pathway
- PMID: 30336783
- PMCID: PMC6193355
- DOI: 10.1186/s13048-018-0462-4
DAXX promotes ovarian cancer ascites cell proliferation and migration by activating the ERK signaling pathway
Abstract
Background: The death-domain-associated protein (DAXX) was originally identified as a protein that binds to the transmembrane death receptor FAS and enhances both FAS-induced and transforming growth factor-β-dependent apoptosis. In a previous study, we found that nude mice injected with DAXX-overexpressing cells (ES-2-DAXX) accumulated large concentrations of first-generation ascites cells (I ascites cells). The role of DAXX in the development of ascites is unknown. The aim of this study was to analyze the effect of DAXX on proliferation and migration of ascites cells in ovarian cancer in vitro and in vivo.
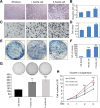
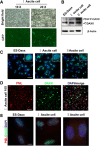
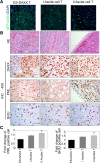
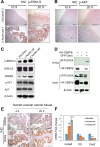
Methods: Nude mice were housed in cages with a 14:10 h light:dark cycle; water and food were provided ad libitum. ES-2-DAXX cells (1×106) were injected intraperitoneally into athymic nude mice (8-week-old female mice). After 4 weeks, I ascites cells were collected. The I ascites cells were injected intraperitoneally into athymic nude mice (8-week-old female mice). After 4 weeks, II ascites cells were collected and cultured. Ascites cell survival, migration, and colony formation were measured using colony formation and cell growth assays. Immunofluorescent staining revealed the co-localization of DAXX and promyelocytic leukemia protein (PML) in ascites cell nuclei. Western blotting and immunohistochemistry showed that extracellular signal-related kinase (p-ERK) 1/2 and CEBP-β were highly expressed in tumor tissues formed by II ascites cells. Through immunoprecipitation, we also found that DAXX can interact with CEBP-β.
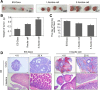
Results: DAXX enhanced ascites cell survival, migration, and colony formation. DAXX and PML nuclear foci dramatically increased in a passage-dependent manner in ascites cells, DAXX promoted the tumor growth of ascites cells in vivo, increased ascites cell proliferation in vivo, and enhanced ascites cell survival and migration by activating the ERK signalling pathway and integrating with CEBP-β.
Conclusions: DAXX can interact with CEBP-β. DAXX can induce ovarian cancer ascites formation by activating the ERK signal pathway and binding to CEBP-β.
Keywords: Ascites cell; Cell migration; Cell proliferation; DAXX.
Conflict of interest statement
Authors’ information
Where the work was performed: Laboratory of molecular biology, college of medicine, Jiaxing University.
Ethics approval
Normal ovaries were provided by the the Jiaxing Maternity and Child Health Care Hospital, China. The use of archived samples in this study was approved by the Jiaxing University Institutional Review Board. The NIH Guides for the Care and Use of Laboratory Animals were used as all animal protocols.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

