Prostate Cancer Screening Patient Decision Aids: A Systematic Review and Meta-analysis
- PMID: 30337235
- PMCID: PMC6467088
- DOI: 10.1016/j.amepre.2018.06.016
Prostate Cancer Screening Patient Decision Aids: A Systematic Review and Meta-analysis
Abstract
Context: Although screening recommendations for prostate cancer using prostate-specific antigen testing often include shared decision making, the effect of patient decision aids on patients' intention and uptake is unclear. This study aimed to review the effect of decision aids on men's screening intention, screening utilization, and the congruence between intentions and uptake.
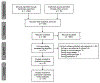
Evidence acquisition: Data sources were searched through April 6, 2018, and included MEDLINE, Scopus, CENTRAL, CT.gov, Cochrane report, PsycARTICLES, PsycINFO, and reference lists. This study included RCTs and observational studies of decision aids that measured prostate screening intention or behavior. The analysis was completed in April 2018.
Evidence synthesis: Eighteen studies (13 RCTs, four before-after studies, and one non-RCT) reported data on screening intention for ≅8,400 men and screening uptake for 2,385 men. Compared with usual care, the use of decision aids in any format results in fewer men (aged ≥40 years) planning to undergo prostate-specific antigen testing (risk ratio=0.88, 95% CI=0.81, 0.95, p=0.006, I2=66%, p<0.001, n=8). Many men did not follow their screening intentions during the first year after using a decision aid; however, most men who were planning to undergo screening did so (probability that men who wanted to be screened would receive screening was 95%).
Conclusions: Integration of decision aids in clinical practice may result in a decrease in the number of men who elect prostate-specific antigen testing, which may in turn reduce screening uptake. To ensure high congruence between intention and screening utilization, providers should not delay the shared decision-making discussion after patients use a decision aid.
Copyright © 2018 American Journal of Preventive Medicine. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No financial disclosures were reported by the authors of this paper.
Figures

References
-
- Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol. 2017;3(4):524–548. 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
-
- SEER Cancer Stat Facts: Prostate Cancer. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/statfacts/html/prost.html. Published 2017. Accessed December 23, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

