STAT3 Inhibition Combined with CpG Immunostimulation Activates Antitumor Immunity to Eradicate Genetically Distinct Castration-Resistant Prostate Cancers
- PMID: 30337279
- PMCID: PMC6279477
- DOI: 10.1158/1078-0432.CCR-18-1277
STAT3 Inhibition Combined with CpG Immunostimulation Activates Antitumor Immunity to Eradicate Genetically Distinct Castration-Resistant Prostate Cancers
Abstract
Purpose: Prostate cancers show remarkable resistance to emerging immunotherapies, partly due to tolerogenic STAT3 signaling in tumor-associated myeloid cells. Here, we describe a novel strategy combining STAT3 inhibition with Toll-like Receptor 9 (TLR9) stimulation to unleash immune response against prostate cancers regardless of the genetic background.
Experimental design: We developed and validated a conjugate of the STAT3 antisense oligonucleotide (ASO) tethered to immunostimulatory TLR9 agonist (CpG oligonucleotide) to improve targeting of human and mouse prostate cancer and myeloid immune cells, such as myeloid-derived suppressor cells (MDSC).
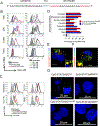
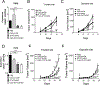
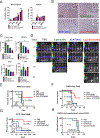
Results: CpG-STAT3ASO conjugates showed improved biodistribution and potency of STAT3 knockdown in target cells in vitro and in vivo. Systemic administration of CpG-STAT3ASO (5 mg/kg) eradicated bone-localized, Ras/Myc-driven, and Ptenpc -/- Smad4pc -/- Trp53c -/- prostate tumors in the majority of treated mice. These antitumor effects were primarily immune-mediated and correlated with an increased ratio of CD8+ to regulatory T cells and reduced pSTAT3+/PD-L1+ MDSCs. Both innate and adaptive immunity contributed to systemic antitumor responses as verified by the depletion of Gr1+ myeloid cells and CD8+ and CD4+ T cells, respectively. Importantly, only the bifunctional CpG-STAT3ASO, but not control CpG oligonucleotides, STAT3ASO alone, or the coinjection of both oligonucleotides, succeeded in recruiting neutrophils and CD8+ T cells into tumors. Thus, the concurrence of TLR9 activation with STAT3 inhibition in the same cellular compartment is indispensable for overcoming tumor immune tolerance and effective antitumor immunity against prostate cancer.
Conclusions: The bifunctional, immunostimulatory, and tolerance-breaking design of CpG-STAT3ASO offers a blueprint for the development of effective and safer oligonucleotide strategies for treatment of immunologically "cold" human cancers.
©2018 American Association for Cancer Research.
Conflict of interest statement
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST
M.K., P.S., D.M. and S.K.P. are inventors on patent application U.S. Provisional Application No.: 62/264,026 submitted by COH that covers the design of oligonucleotides presented in this report. All other authors declare no potential conflict of interest.
Figures



Similar articles
-
Myeloid cells as a target for oligonucleotide therapeutics: turning obstacles into opportunities.Cancer Immunol Immunother. 2017 Aug;66(8):979-988. doi: 10.1007/s00262-017-1966-2. Epub 2017 Feb 18. Cancer Immunol Immunother. 2017. PMID: 28214929 Free PMC article. Review.
-
B Cell Lymphoma Immunotherapy Using TLR9-Targeted Oligonucleotide STAT3 Inhibitors.Mol Ther. 2018 Mar 7;26(3):695-707. doi: 10.1016/j.ymthe.2018.01.007. Epub 2018 Jan 17. Mol Ther. 2018. PMID: 29433938 Free PMC article.
-
Leukemia cell-targeted STAT3 silencing and TLR9 triggering generate systemic antitumor immunity.Blood. 2014 Jan 2;123(1):15-25. doi: 10.1182/blood-2013-07-517987. Epub 2013 Oct 29. Blood. 2014. PMID: 24169824 Free PMC article.
-
TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients.Clin Cancer Res. 2015 Aug 15;21(16):3771-82. doi: 10.1158/1078-0432.CCR-14-3145. Epub 2015 May 12. Clin Cancer Res. 2015. PMID: 25967142 Free PMC article.
-
STAT3 in Tumor-Associated Myeloid Cells: Multitasking to Disrupt Immunity.Int J Mol Sci. 2018 Jun 19;19(6):1803. doi: 10.3390/ijms19061803. Int J Mol Sci. 2018. PMID: 29921770 Free PMC article. Review.
Cited by
-
Pharmacological modulation of myeloid-derived suppressor cells to dampen inflammation.Front Immunol. 2022 Aug 30;13:933847. doi: 10.3389/fimmu.2022.933847. eCollection 2022. Front Immunol. 2022. PMID: 36110844 Free PMC article. Review.
-
Application of Anti-Inflammatory Agents in Prostate Cancer.J Clin Med. 2020 Aug 18;9(8):2680. doi: 10.3390/jcm9082680. J Clin Med. 2020. PMID: 32824865 Free PMC article. Review.
-
Targeted TLR9 Agonist Elicits Effective Antitumor Immunity against Spontaneously Arising Breast Tumors.J Immunol. 2023 Jul 15;211(2):295-305. doi: 10.4049/jimmunol.2200950. J Immunol. 2023. PMID: 37256255 Free PMC article.
-
Myeloid cell-targeted STAT3 inhibition sensitizes head and neck cancers to radiotherapy and T cell-mediated immunity.J Clin Invest. 2021 Jan 19;131(2):e137001. doi: 10.1172/JCI137001. J Clin Invest. 2021. PMID: 33232304 Free PMC article.
-
Generation of Myeloid Cells in Cancer: The Spleen Matters.Front Immunol. 2020 Jun 5;11:1126. doi: 10.3389/fimmu.2020.01126. eCollection 2020. Front Immunol. 2020. PMID: 32582203 Free PMC article. Review.
References
-
- Miyahira AK, Kissick HT, Bishop JL, Takeda DY, Barbieri CE, Simons JW, et al. Beyond immune checkpoint blockade: new approaches to targeting host-tumor interactions in prostate cancer: report from the 2014 Coffey-Holden prostate cancer academy meeting. Prostate. 2015;75:337–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

