Osteogenic niche in the regulation of normal hematopoiesis and leukemogenesis
- PMID: 30337364
- PMCID: PMC6269284
- DOI: 10.3324/haematol.2018.197004
Osteogenic niche in the regulation of normal hematopoiesis and leukemogenesis
Abstract
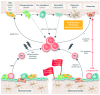
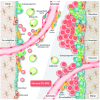
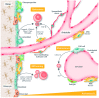
The bone marrow microenvironment, also known as the bone marrow niche, is a complex network of cell types and acellular factors that supports normal hematopoiesis. For many years, leukemia was believed to be caused by a series of genetic hits to hematopoietic stem and progenitor cells, which transform them to preleukemic, and eventually to leukemic, cells. Recent discoveries suggest that genetic alterations in bone marrow niche cells, particularly in osteogenic cells, may also cause myeloid leukemia in mouse models. The osteogenic niche, which consists of osteoprogenitors, preosteoblasts, mature osteoblasts, osteocytes and osteoclasts, has been shown to play a critical role in the maintenance and expansion of hematopoietic stem and progenitor cells as well as in their oncogenic transformation into leukemia stem/initiating cells. We have recently shown that acute myeloid leukemia cells induce osteogenic differentiation in mesenchymal stromal cells to gain a growth advantage. In this review, we discuss the role of the osteogenic niche in the maintenance of hematopoietic stem and progenitor cells, as well as in their transformation into leukemia cells. We also discuss the signaling pathways that regulate osteogenic niche-hematopoietic stem and progenitor cells or osteogenic niche-leukemic stem/initiating cell interactions in the bone marrow, together with novel approaches for therapeutically targeting these interactions.
Copyright© 2018 Ferrata Storti Foundation.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

