Evidence that a respiratory shield in Escherichia coli protects a low-molecular-mass FeII pool from O2-dependent oxidation
- PMID: 30337367
- PMCID: PMC6322884
- DOI: 10.1074/jbc.RA118.005233
Evidence that a respiratory shield in Escherichia coli protects a low-molecular-mass FeII pool from O2-dependent oxidation
Abstract
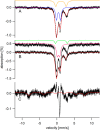
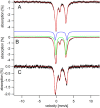
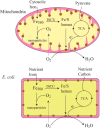
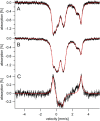
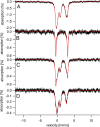
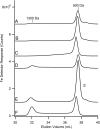
Iron is critical for virtually all organisms, yet major questions remain regarding the systems-level understanding of iron in whole cells. Here, we obtained Mössbauer and EPR spectra of Escherichia coli cells prepared under different nutrient iron concentrations, carbon sources, growth phases, and O2 concentrations to better understand their global iron content. We investigated WT cells and those lacking Fur, FtnA, Bfr, and Dps proteins. The coarse-grain iron content of exponentially growing cells consisted of iron-sulfur clusters, variable amounts of nonheme high-spin FeII species, and an unassigned residual quadrupole doublet. The iron in stationary-phase cells was dominated by magnetically ordered FeIII ions due to oxyhydroxide nanoparticles. Analysis of cytosolic extracts by size-exclusion chromatography detected by an online inductively coupled plasma mass spectrometer revealed a low-molecular-mass (LMM) FeII pool consisting of two iron complexes with masses of ∼500 (major) and ∼1300 (minor) Da. They appeared to be high-spin FeII species with mostly oxygen donor ligands, perhaps a few nitrogen donors, and probably no sulfur donors. Surprisingly, the iron content of E. coli and its reactivity with O2 were remarkably similar to those of mitochondria. In both cases, a "respiratory shield" composed of membrane-bound iron-rich respiratory complexes may protect the LMM FeII pool from reacting with O2 When exponentially growing cells transition to stationary phase, the shield deactivates as metabolic activity declines. Given the universality of oxidative phosphorylation in aerobic biology, the iron content and respiratory shield in other aerobic prokaryotes might be similar to those of E. coli and mitochondria.
Keywords: Mössbauer spectroscopy; chemiosmotic coupling; cyanide; electron paramagnetic resonance (EPR); ferric uptake regulator; ferritin; iron homeostasis; iron metabolism; labile iron pool; metal homeostasis; mitochondria.
© 2019 Wofford et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Gütlich P., Bill E., and Trautwein A. X. (2011) Mössbauer Spectroscopy and Transition Metal Chemistry, Springer, New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

