Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis
- PMID: 30337409
- PMCID: PMC6218007
- DOI: 10.1126/science.aau3301
Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis
Abstract
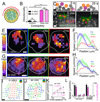
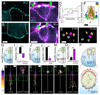
Collective cell chemotaxis, the directed migration of cell groups along gradients of soluble chemical cues, underlies various developmental and pathological processes. We use neural crest cells, a migratory embryonic stem cell population whose behavior has been likened to malignant invasion, to study collective chemotaxis in vivo. Studying Xenopus and zebrafish, we have shown that the neural crest exhibits a tensile actomyosin ring at the edge of the migratory cell group that contracts in a supracellular fashion. This contractility is polarized during collective cell chemotaxis: It is inhibited at the front but persists at the rear of the cell cluster. The differential contractility drives directed collective cell migration ex vivo and in vivo through the intercalation of rear cells. Thus, in neural crest cells, collective chemotaxis works by rear-wheel drive.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
Supracellular contractions propel migration.Science. 2018 Oct 19;362(6412):290-291. doi: 10.1126/science.aav3376. Science. 2018. PMID: 30337397 No abstract available.
References
-
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nature Reviews Molecular Cell Biology. 2009;10:445–457. - PubMed
-
- Roca-Cusachs P, Sunyer R, Trepat X. Mechanical guidance of cell migration: lessons from chemotaxis. Current Opinion in Cell Biology. 2013;25:543–549. - PubMed
-
- Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nature Reviews Molecular Cell Biology. 2016;17:97–109. - PubMed
-
- Haeger A, Wolf K, Zegers MM, Friedl P. Collective cell migration: guidance principles and hierarchies. Trends in Cell Biology. 2015;25:556–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

