Unraveling the role of B cells in the pathogenesis of an oncogenic avian herpesvirus
- PMID: 30337483
- PMCID: PMC6233081
- DOI: 10.1073/pnas.1813964115
Unraveling the role of B cells in the pathogenesis of an oncogenic avian herpesvirus
Abstract
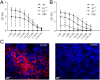
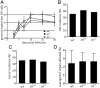
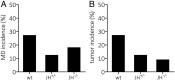
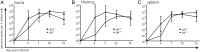
Marek's disease virus (MDV) is a highly oncogenic alphaherpesvirus that causes immunosuppression, paralysis, and deadly lymphomas in chickens. In infected animals, B cells are efficiently infected and are thought to amplify the virus and transfer it to T cells. MDV subsequently establishes latency in T cells and transforms CD4+ T cells, resulting in fatal lymphomas. Despite many years of research, the exact role of the different B and T cell subsets in MDV pathogenesis remains poorly understood, mostly due to the lack of reverse genetics in chickens. Recently, Ig heavy chain J gene segment knockout (JH-KO) chickens lacking mature and peripheral B cells have been generated. To determine the role of these B cells in MDV pathogenesis, we infected JH-KO chickens with the very virulent MDV RB1B strain. Surprisingly, viral load in the blood of infected animals was not altered in the absence of B cells. More importantly, disease and tumor incidence in JH-KO chickens was comparable to wild-type animals, suggesting that both mature and peripheral B cells are dispensable for MDV pathogenesis. Intriguingly, MDV efficiently replicated in the bursa of Fabricius in JH-KO animals, while spread of the virus to the spleen and thymus was delayed. In the absence of B cells, MDV readily infected CD4+ and CD8+ T cells, allowing efficient virus replication in the lymphoid organs and transformation of T cells. Taken together, our data change the dogma of the central role of B cells, and thereby provide important insights into MDV pathogenesis.
Keywords: B cells; Ig knockout chickens; Marek’s disease virus; lymphomagenesis; transgenic chickens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Unraveling the role of γδ T cells in the pathogenesis of an oncogenic avian herpesvirus.mBio. 2024 Aug 14;15(8):e0031524. doi: 10.1128/mbio.00315-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953352 Free PMC article.
-
Induction of DNA Damages upon Marek's Disease Virus Infection: Implication in Viral Replication and Pathogenesis.J Virol. 2017 Nov 30;91(24):e01658-17. doi: 10.1128/JVI.01658-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978699 Free PMC article.
-
Relationship between the immunosuppressive potential and the pathotype of Marek's disease virus isolates.Avian Dis. 1998 Jan-Mar;42(1):124-32. Avian Dis. 1998. PMID: 9533089
-
Revisiting cellular immune response to oncogenic Marek's disease virus: the rising of avian T-cell immunity.Cell Mol Life Sci. 2020 Aug;77(16):3103-3116. doi: 10.1007/s00018-020-03477-z. Epub 2020 Feb 20. Cell Mol Life Sci. 2020. PMID: 32080753 Free PMC article. Review.
-
Latest Insights into Unique Open Reading Frames Encoded by Unique Long (UL) and Short (US) Regions of Marek's Disease Virus.Viruses. 2021 May 25;13(6):974. doi: 10.3390/v13060974. Viruses. 2021. PMID: 34070255 Free PMC article. Review.
Cited by
-
Visualization of Marek's Disease Virus Genomes in Living Cells during Lytic Replication and Latency.Viruses. 2022 Jan 29;14(2):287. doi: 10.3390/v14020287. Viruses. 2022. PMID: 35215880 Free PMC article.
-
Marek's Disease Virus Infection of Natural Killer Cells.Microorganisms. 2019 Nov 20;7(12):588. doi: 10.3390/microorganisms7120588. Microorganisms. 2019. PMID: 31757008 Free PMC article.
-
Characterization of a Novel Viral Interleukin 8 (vIL-8) Splice Variant Encoded by Marek's Disease Virus.Microorganisms. 2021 Jul 9;9(7):1475. doi: 10.3390/microorganisms9071475. Microorganisms. 2021. PMID: 34361910 Free PMC article.
-
Abrogation of Marek's disease virus replication using CRISPR/Cas9.Sci Rep. 2020 Jul 2;10(1):10919. doi: 10.1038/s41598-020-67951-1. Sci Rep. 2020. PMID: 32616820 Free PMC article.
-
Unraveling the role of γδ T cells in the pathogenesis of an oncogenic avian herpesvirus.mBio. 2024 Aug 14;15(8):e0031524. doi: 10.1128/mbio.00315-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953352 Free PMC article.
References
-
- Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek’s disease virus: From miasma to model. Nat Rev Microbiol. 2006;4:283–294. - PubMed
-
- Nair V. Evolution of Marek’s disease–A paradigm for incessant race between the pathogen and the host. Vet J. 2005;170:175–183. - PubMed
-
- Parcells MS, Burnside J, Morgan RW. 2012. Marek’s disease virus-induced T-cell lymphomas. Cancer Associated Viruses. Current Cancer Research (Springer, New York), pp 307–335.
-
- Sharma JM, Witter RL. The effect of B-cell immunosuppression on age-related resistance of chickens to Marek’s disease. Cancer Res. 1975;35:711–717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

