Non-invasive continuous real-time in vivo analysis of microbial hydrogen production shows adaptation to fermentable carbohydrates in mice
- PMID: 30337551
- PMCID: PMC6193968
- DOI: 10.1038/s41598-018-33619-0
Non-invasive continuous real-time in vivo analysis of microbial hydrogen production shows adaptation to fermentable carbohydrates in mice
Abstract
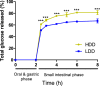
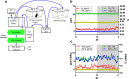
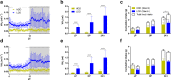
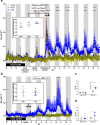
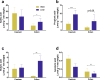
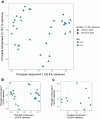
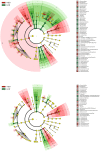
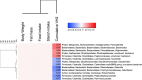
Real time in vivo methods are needed to better understand the interplay between diet and the gastrointestinal microbiota. Therefore, a rodent indirect calorimetry system was equipped with hydrogen (H2) and methane (CH4) sensors. H2 production was readily detected in C57BL/6J mice and followed a circadian rhythm. H2 production was increased within 12 hours after first exposure to a lowly-digestible starch diet (LDD) compared to a highly-digestible starch diet (HDD). Marked differences were observed in the faecal microbiota of animals fed the LDD and HDD diets. H2 was identified as a key variable explaining the variation in microbial communities, with specific taxa (including Bacteroides and Parasutterella) correlating with H2 production upon LDD-feeding. CH4 production was undetectable which was in line with absence of CH4 producers in the gut. We conclude that real-time in vivo monitoring of gases provides a non-invasive time-resolved system to explore the interplay between nutrition and gut microbes in a mouse model, and demonstrates potential for translation to other animal models and human studies.
Conflict of interest statement
N.B. and V.G.-C. are employed by Cargill. A.O. is employed by Danone Nutricia Research.
Figures
Similar articles
-
Intestinal transport and fermentation of resistant starch evaluated by the hydrogen breath test.Eur J Clin Nutr. 1994 Oct;48(10):692-701. Eur J Clin Nutr. 1994. PMID: 7835324 Clinical Trial.
-
Influence of menstrual cycle and oral contraception on taxonomic composition and gas production in the gut microbiome.J Med Microbiol. 2025 Mar;74(3):001987. doi: 10.1099/jmm.0.001987. J Med Microbiol. 2025. PMID: 40153295 Free PMC article.
-
Circadian patterns of total 24-h hydrogen and methane excretion in humans ingesting nonstarch polysaccharide (NSP) diets and the implications for indirect calorimetric and D2 18O methodologies.Eur J Clin Nutr. 1996 Aug;50(8):524-34. Eur J Clin Nutr. 1996. PMID: 8863013
-
Hydrogen and methane breath tests for evaluation of resistant carbohydrates.Eur J Clin Nutr. 1992 Oct;46 Suppl 2:S77-90. Eur J Clin Nutr. 1992. PMID: 1330532 Review.
-
Effects of three methane mitigation agents on parameters of kinetics of total and hydrogen gas production, ruminal fermentation and hydrogen balance using in vitro technique.Anim Sci J. 2016 Feb;87(2):224-32. doi: 10.1111/asj.12423. Epub 2015 Jul 29. Anim Sci J. 2016. PMID: 26223853 Review.
Cited by
-
Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms.Microorganisms. 2020 Mar 13;8(3):406. doi: 10.3390/microorganisms8030406. Microorganisms. 2020. PMID: 32183143 Free PMC article.
-
Effects of Zymosan on Short-Chain Fatty Acid and Gas Production in in vitro Fermentation Models of the Human Intestinal Microbiota.Front Nutr. 2022 Jul 4;9:921137. doi: 10.3389/fnut.2022.921137. eCollection 2022. Front Nutr. 2022. PMID: 35859755 Free PMC article.
-
An ethically guided preclinical device for phenotyping H2 production in laboratory rodents.Animal Model Exp Med. 2024 Aug;7(4):553-561. doi: 10.1002/ame2.12460. Epub 2024 Jun 25. Animal Model Exp Med. 2024. PMID: 38925626 Free PMC article.
-
Galactose in the Post-Weaning Diet Programs Improved Circulating Adiponectin Concentrations and Skeletal Muscle Insulin Signaling.Int J Mol Sci. 2022 Sep 6;23(18):10207. doi: 10.3390/ijms231810207. Int J Mol Sci. 2022. PMID: 36142131 Free PMC article.
-
The Effect of Dietary Supplementation with Resveratrol on Growth Performance, Carcass and Meat Quality, Blood Lipid Levels and Ruminal Microbiota in Fattening Goats.Foods. 2022 Feb 18;11(4):598. doi: 10.3390/foods11040598. Foods. 2022. PMID: 35206074 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

