Surfactant protein D attenuates acute lung and kidney injuries in pneumonia-induced sepsis through modulating apoptosis, inflammation and NF-κB signaling
- PMID: 30337682
- PMCID: PMC6193952
- DOI: 10.1038/s41598-018-33828-7
Surfactant protein D attenuates acute lung and kidney injuries in pneumonia-induced sepsis through modulating apoptosis, inflammation and NF-κB signaling
Abstract
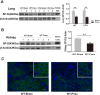
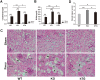
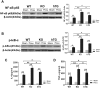
Pneumonia and sepsis are major risk factors for acute kidney injury (AKI). Patients with pneumonia and AKI are at increased risk for morbidity and mortality. Surfactant protein D (SP-D) expressed in lung and kidney plays important roles in innate immunity. However, little is known about the role of organ-specific SP-D in the sepsis. The current study uses wild type (WT), SP-D knockout (KO), and humanized SP-D transgenic (hTG, lung-specific SP-D expression) mice to study organ-specific role of SP-D in pneumonia-induced sepsis. Analyses demonstrated differential lung and kidney injury among three-type mice infected with Pseudomonas aeruginosa. After infection, KO mice showed higher injurious scores in both lung and kidney, and decreased renal function than WT and hTG mice. hTG mice exhibited comparable lung injury but more severe kidney injury compared to WT mice. Increased renal tubular apoptosis, NF-κB activation and proinflammatory cytokines in the kidney of KO mice were found when compared with WT and hTG mice. Furthermore, in vitro primary proximal tubular epithelial cells from KO mice showed more apoptosis with higher level of activated caspase-3 than those from WT mice after LPS treatment. Collectively, SP-D attenuates AKI in the sepsis by modulating renal apoptosis, inflammation and NF-κB signaling.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

