Calcium in the Pathophysiology of Atrial Fibrillation and Heart Failure
- PMID: 30337881
- PMCID: PMC6180171
- DOI: 10.3389/fphys.2018.01380
Calcium in the Pathophysiology of Atrial Fibrillation and Heart Failure
Abstract
Atrial fibrillation (AF) is commonly associated with heart failure. A bidirectional relationship exists between the two-AF exacerbates heart failure causing a significant increase in heart failure symptoms, admissions to hospital and cardiovascular death, while pathological remodeling of the atria as a result of heart failure increases the risk of AF. A comprehensive understanding of the pathophysiology of AF is essential if we are to break this vicious circle. In this review, the latest evidence will be presented showing a fundamental role for calcium in both the induction and maintenance of AF. After outlining atrial electrophysiology and calcium handling, the role of calcium-dependent afterdepolarizations and atrial repolarization alternans in triggering AF will be considered. The atrial response to rapid stimulation will be discussed, including the short-term protection from calcium overload in the form of calcium signaling silencing and the eventual progression to diastolic calcium leak causing afterdepolarizations and the development of an electrical substrate that perpetuates AF. The role of calcium in the bidirectional relationship between heart failure and AF will then be covered. The effects of heart failure on atrial calcium handling that promote AF will be reviewed, including effects on both atrial myocytes and the pulmonary veins, before the aspects of AF which exacerbate heart failure are discussed. Finally, the limitations of human and animal studies will be explored allowing contextualization of what are sometimes discordant results.
Keywords: atrial fibrillation; calcium; heart failure; pathophysiology; t-tubules.
Figures
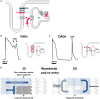
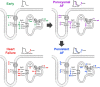
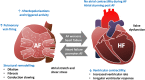
References
-
- Allessie M. A., Bonke F. I., Schopman F. J. (1977). Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ. Res. 41, 9–18. 10.1161/01.RES.41.1.9 - DOI - PubMed
Publication types
Grants and funding
- FS/12/57/29717/BHF_/British Heart Foundation/United Kingdom
- FS/12/34/29565/BHF_/British Heart Foundation/United Kingdom
- FS/09/002/26487/BHF_/British Heart Foundation/United Kingdom
- PG/12/89/29970/BHF_/British Heart Foundation/United Kingdom
- FS/17/54/33126/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources

