Nanoparticle Vaccines Against Infectious Diseases
- PMID: 30337923
- PMCID: PMC6180194
- DOI: 10.3389/fimmu.2018.02224
Nanoparticle Vaccines Against Infectious Diseases
Abstract
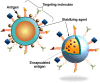
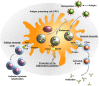
Due to emergence of new variants of pathogenic micro-organisms the treatment and immunization of infectious diseases have become a great challenge in the past few years. In the context of vaccine development remarkable efforts have been made to develop new vaccines and also to improve the efficacy of existing vaccines against specific diseases. To date, some vaccines are developed from protein subunits or killed pathogens, whilst several vaccines are based on live-attenuated organisms, which carry the risk of regaining their pathogenicity under certain immunocompromised conditions. To avoid this, the development of risk-free effective vaccines in conjunction with adequate delivery systems are considered as an imperative need to obtain desired humoral and cell-mediated immunity against infectious diseases. In the last several years, the use of nanoparticle-based vaccines has received a great attention to improve vaccine efficacy, immunization strategies, and targeted delivery to achieve desired immune responses at the cellular level. To improve vaccine efficacy, these nanocarriers should protect the antigens from premature proteolytic degradation, facilitate antigen uptake and processing by antigen presenting cells, control release, and should be safe for human use. Nanocarriers composed of lipids, proteins, metals or polymers have already been used to attain some of these attributes. In this context, several physico-chemical properties of nanoparticles play an important role in the determination of vaccine efficacy. This review article focuses on the applications of nanocarrier-based vaccine formulations and the strategies used for the functionalization of nanoparticles to accomplish efficient delivery of vaccines in order to induce desired host immunity against infectious diseases.
Keywords: antigens; human diseases; nanoparticles; targeted vaccine delivery; vaccine development.
Figures
References
-
- WHO Global Tuberculosis Report. World Health Organization (2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

