Does clinical outcome of birch pollen immunotherapy relate to induction of blocking antibodies preventing IgE from allergen binding? A pilot study monitoring responses during first year of AIT
- PMID: 30338052
- PMCID: PMC6174570
- DOI: 10.1186/s13601-018-0226-7
Does clinical outcome of birch pollen immunotherapy relate to induction of blocking antibodies preventing IgE from allergen binding? A pilot study monitoring responses during first year of AIT
Abstract
Background: The clinical benefit of allergen-specific immunotherapy (AIT) involves induction of blocking antibodies. It is not clear if these antibodies function via steric hindrance alone or a combination of levels, avidities, and epitope specificities, and clinical outcome cannot be predicted. We aim to in-depth characterize serum antibody profiles during birch pollen AIT, investigate therapy-induced antibodies for their capacity to block IgE binding to Bet v 1 and correlate data with clinical outcomes.
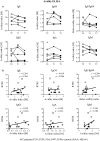
Methods: Immune responses of five birch pollen allergic patients were monitored during the first year of AIT by nasal provocation tests (NPTs), ImmunoCAP, immunoblots, direct and avidity enzyme-linked immunosorbent assays, mediator release assays, facilitated antigen binding (FAB) assays, and inhibition mediator release assays.
Results: There was no correlation between NPT results and therapy-induced changes in levels (IgE, IgG, IgA, IgM), avidities, or mediator release potency of Bet v 1-specific antibodies. In FAB assays, blocking antibodies initiated upon AIT were shown to prevent formation of Bet v 1-IgE complexes of an indicator serum pool and significantly correlated with clinical readout. Inhibition mediator release assays using patient-specific IgE for passive sensitization revealed therapy-induced blocking capacities with very good correlation to NPT results. Notably, this assay was the only one to detect a non-responder during treatment in this pilot study.
Conclusions: Clinical outcome of AIT depends on induction of blocking antibodies able to prevent the patient's own IgE from allergen binding. Monitoring of clinical efficacy seems to be best achieved using the inhibition mediator release assay, as development of relevant blocking antibodies can be verified in a patient-tailored manner.
Keywords: Allergens and epitopes; Bet v 1; Birch pollen; Blocking capacity; FAB assay; IgE; Immunotherapy; Inhibition mediator release assay.
Figures






References
-
- EAACI White Paper on Research, Innovation and Quality Care. Zurich, Switzerland: European Academy of Allergy and Clinical Immunology; 2018. http://www.eaaci.org/documents/EAACI_White_Paper.pdf.
-
- Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, Nelson H, Akdis CA. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(1288–1296):e1283. - PubMed
-
- James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, Jacobson MR, Kimber I, Till SJ, Durham SR. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127:509–516. doi: 10.1016/j.jaci.2010.12.1080. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

