Wearable speckle plethysmography (SPG) for characterizing microvascular flow and resistance
- PMID: 30338166
- PMCID: PMC6191642
- DOI: 10.1364/BOE.9.003937
Wearable speckle plethysmography (SPG) for characterizing microvascular flow and resistance
Abstract
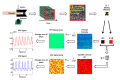
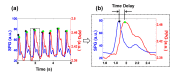
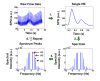
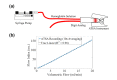
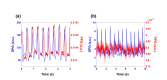
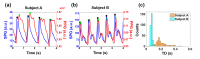
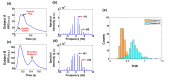
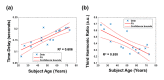
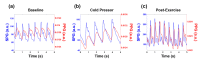
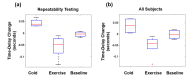
In this work we introduce a modified form of laser speckle imaging (LSI) referred to as affixed transmission speckle analysis (ATSA) that uses a single coherent light source to probe two physiological signals: one related to pulsatile vascular expansion (classically known as the photoplethysmographic (PPG) waveform) and one related to pulsatile vascular blood flow (named here the speckle plethysmographic (SPG) waveform). The PPG signal is determined by recording intensity fluctuations, and the SPG signal is determined via the LSI dynamic light scattering technique. These two co-registered signals are obtained by transilluminating a single digit (e.g. finger) which produces quasi-periodic waveforms derived from the cardiac cycle. Because PPG and SPG waveforms probe vascular expansion and flow, respectively, in cm-thick tissue, these complementary phenomena are offset in time and have rich dynamic features. We characterize the timing offset and harmonic content of the waveforms in 16 human subjects and demonstrate physiologic relevance for assessing microvascular flow and resistance.
Keywords: (170.0170) Medical optics and biotechnology; (170.3890) Medical optics instrumentation; (230.0230) Optical devices.
Conflict of interest statement
MG: University of California, Irvine (P), TBR: LAS Inc. (I, E, P), BY: LAS Inc. (I, E, P), SMW: LAS Inc. (I, E, P), BJT University of California, Irvine (P).
Figures

References
-
- Webster J. G., Design of pulse oximeters (CRC Press, 1997).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
