Rosiglitazone suppresses RANKL-induced NFATc1 autoamplification by disrupting the physical interaction between NFATc1 and PPARγ
- PMID: 30338210
- PMCID: PMC6168694
- DOI: 10.1002/2211-5463.12513
Rosiglitazone suppresses RANKL-induced NFATc1 autoamplification by disrupting the physical interaction between NFATc1 and PPARγ
Abstract
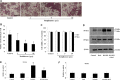
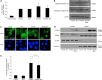
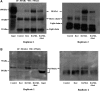
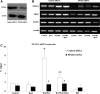
Receptor activator of nuclear factor-κB ligand (RANKL) is required for initiation of osteoclastogenesis, with the signaling pathway including the NF-kB, c-Fos, and nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) transcription factors. Because NFATc1 expression is autoamplified, we investigated the molecular mechanism by which peroxisome proliferator-activated receptor gamma (PPARγ) activation by the thiazolidinedione drug rosiglitazone decreases NFATc1 expression during RANKL stimulation. Western blotting demonstrated that rosiglitazone attenuated the increase in NFATc1 protein level induced by RANKL without affecting that of PPARγ. Immunofluorescence data indicated that rosiglitazone tended to suppress RANKL-induced NFATc1 nuclear translocation, partly by reducing calcineurin activity, as reflected by the observed decrease in nuclear NFATc1 abundance. On coimmunoprecipitation, the intensity of the physical interaction between NFATc1 and PPARγ was unexpectedly higher in the RANKL-stimulated group than in the control, but rosiglitazone reduced this to basal levels. Furthermore, RANKL failed to elevate mRNA expression of NFATc1 after PPARγ knockdown. ChIP assay indicated that rosiglitazone significantly reduced the binding of NFATc1 to its own promoter despite RANKL stimulation. These findings suggest that PPARγ activation by rosiglitazone blocks NFATc1 from binding to its own promoter, thereby reducing RANKL-induced NFATc1 autoamplification.
Keywords: NFATc1; PPARγ; osteoclastogenesis; physical interaction; rosiglitazone.
Figures
Similar articles
-
n-3 polyunsaturated fatty acids stimulate osteoclastogenesis through PPARγ-mediated enhancement of c-Fos expression, and suppress osteoclastogenesis through PPARγ-dependent inhibition of NFkB activation.J Nutr Biochem. 2015 Nov;26(11):1317-27. doi: 10.1016/j.jnutbio.2015.06.007. Epub 2015 Jul 26. J Nutr Biochem. 2015. PMID: 26303404
-
Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253
-
Trapidil, a platelet-derived growth factor antagonist, inhibits osteoclastogenesis by down-regulating NFATc1 and suppresses bone loss in mice.Biochem Pharmacol. 2013 Sep 15;86(6):782-90. doi: 10.1016/j.bcp.2013.07.015. Epub 2013 Aug 6. Biochem Pharmacol. 2013. PMID: 23928189
-
High extracellular calcium-induced NFATc3 regulates the expression of receptor activator of NF-κB ligand in osteoblasts.Bone. 2011 Aug;49(2):242-9. doi: 10.1016/j.bone.2011.04.006. Epub 2011 Apr 14. Bone. 2011. PMID: 21514407
-
The molecular understanding of osteoclast differentiation.Bone. 2007 Feb;40(2):251-64. doi: 10.1016/j.bone.2006.09.023. Epub 2006 Nov 13. Bone. 2007. PMID: 17098490 Review.
Cited by
-
Lonafarnib Inhibits Farnesyltransferase via Suppressing ERK Signaling Pathway to Prevent Osteoclastogenesis in Titanium Particle-Induced Osteolysis.Front Pharmacol. 2022 Mar 1;13:848152. doi: 10.3389/fphar.2022.848152. eCollection 2022. Front Pharmacol. 2022. PMID: 35300293 Free PMC article.
-
Peroxisome Proliferator-Activated Receptor-γ Antagonizes LOX-1-Mediated Endothelial Injury by Transcriptional Activation of miR-590-5p.PPAR Res. 2019 Jul 1;2019:2715176. doi: 10.1155/2019/2715176. eCollection 2019. PPAR Res. 2019. PMID: 31354796 Free PMC article.
-
The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases.Front Immunol. 2021 Mar 31;12:664871. doi: 10.3389/fimmu.2021.664871. eCollection 2021. Front Immunol. 2021. PMID: 33868316 Free PMC article. Review.
References
-
- Wei S, Teitelbaum SL, Wang MW and Ross FP (2001) Receptor activator of nuclear factor‐kappa b ligand activates nuclear factor‐kappa b in osteoclast precursors. Endocrinology 142, 1290–1295. - PubMed
-
- Hirotani H, Tuohy NA, Woo JT, Stern PH and Clipstone NA (2004) The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem 279, 13984–13992. - PubMed
-
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J et al (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3, 889–901. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

