The C-terminal segment of collagenase in Grimontia hollisae binds collagen to enhance collagenolysis
- PMID: 30338219
- PMCID: PMC6168687
- DOI: 10.1002/2211-5463.12510
The C-terminal segment of collagenase in Grimontia hollisae binds collagen to enhance collagenolysis
Abstract
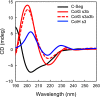
The collagenase secreted by Grimontia hollisae strain 1706B is a 74 kDa protein that consists of two parts: the catalytic module and a C-terminal segment that includes the bacterial pre-peptidase C-terminal domain. Here, we produced a recombinant C-terminal segment protein and examined its ability to bind collagen and other characteristics as compared with collagen-binding domains (CBDs) derived from Hathewaya histolytica (Clostridium histolyticum) collagenases; these CBDs are the only ones thus far identified in bacterial collagenases. We found that the C-terminal segment binds to collagen only when the collagen is in its triple-helical conformation. Moreover, the C-terminal segment and the CBDs from H. histolytica have comparable characteristics, including binding affinity to type I collagen, substrate spectrum, and binding conditions with respect to salt concentration and pH. However, the C-terminal segment has a completely different primary structure from those of the CBDs from H. histolytica. As regards secondary structure, in silico prediction indicates that the C-terminal segment may be homologous to those in CBDs from H. histolytica. Furthermore, we performed collagenase assays using fluorescein isothiocyanate-labeled type I collagen to show that the C-terminal segment positively contributes to the collagenolytic activity of the 74 kDa collagenase from G. hollisae.
Keywords: Grimontia hollisae; PPC domain; bacterial collagenase; metallopeptidase M9 subfamily A; recombinant protein; triple‐helical conformation.
Figures







References
-
- Thompson FL, Hoste B, Vandemeulebroecke K and Swings J (2003) Reclassification of Vibrio hollisae as Grimontia hollisae gen. nov., comb. nov. Int J Syst Evol Microbiol 53, 1615–1617. - PubMed
-
- Suzuki K (2000) Purification and properties of collagenase from Vibrio hollisae 1706B strain. Hikakukagaku. 45, 272–283.
-
- Lawson PA and Rainey FA (2016) Proposal to restrict the genus Clostridium Prazmowski to Clostridium butyricum and related species. Int J Syst Evol Microbiol 66, 1009–1016. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

