Infant formulas containing hydrolysed protein for prevention of allergic disease
- PMID: 30338526
- PMCID: PMC6517017
- DOI: 10.1002/14651858.CD003664.pub6
Infant formulas containing hydrolysed protein for prevention of allergic disease
Abstract
Background: Infant formulas containing hydrolysed proteins have been widely advocated for preventing allergic disease in infants, in place of standard cow's milk formula (CMF). However, it is unclear whether the clinical trial evidence supports this.
Objectives: To compare effects on allergic disease when infants are fed a hydrolysed formula versus CMF or human breast milk. If hydrolysed formulas are effective, to determine what type of hydrolysed formula is most effective, including extensively or partially hydrolysed formula (EHF/PHF). To determine whether infants at low or high risk of allergic disease, and whether infants receiving early short-term (first few days after birth) or prolonged formula feeding benefit from hydrolysed formulas.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 11), MEDLINE (1948 to 3 November 2017), and Embase (1974 to 3 November 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles and previous reviews for randomised controlled trials and quasi-randomised trials.
Selection criteria: We searched for randomised and quasi-randomised trials that compared use of a hydrolysed formula versus human milk or CMF. Outcomes with ≥ 80% follow-up of participants from eligible trials were eligible for inclusion.
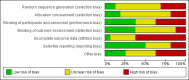
Data collection and analysis: Two review authors independently selected trials, assessed trial quality and extracted data from the included studies. Fixed-effect analyses were performed. The treatment effects were expressed as risk ratio (RR) and risk difference (RD) with 95% confidence intervals and quality of evidence using the GRADE quality of evidence approach. The primary outcome was all allergic disease (including asthma, atopic dermatitis, allergic rhinitis and food allergy).
Main results: A total of 16 studies were included.Two studies assessed the effect of three to four days infant supplementation with an EHF while in hospital after birth versus pasteurised human milk feed. A single study enrolling 90 infants reported no difference in all allergic disease (RR 1.43, 95% CI 0.38 to 5.37) or any specific allergic disease up to childhood including cow's milk allergy (CMA) (RR 7.11, 95% CI 0.35 to 143.84). A single study reported no difference in infant CMA (RR 0.87, 95% CI 0.52 to 1.46; participants = 3559). Quality of evidence was assessed as very low for all outcomes.No eligible trials compared prolonged hydrolysed formula versus human milk feeding.Two studies assessed the effect of three to four days infant supplementation with an EHF versus a CMF. A single study enrolling 90 infants reported no difference in all allergic disease (RR 1.37, 95% CI 0.33 to 5.71; participants = 77) or any specific allergic disease including CMA up to childhood. A single study reported a reduction in infant CMA of borderline significance (RR 0.62, 95% CI 0.38 to 1.00; participants = 3473). Quality of evidence was assessed as very low for all outcomes.Twelve studies assessed the effect of prolonged infant feeding with a hydrolysed formula compared with a CMF. The data showed no difference in all allergic disease in infants (typical RR 0.88, 95% CI 0.76 to 1.01; participants = 2852; studies = 8) and children (typical RR 0.85, 95% CI 0.69 to 1.05; participants = 950; studies = 2), and no difference in any specific allergic disease including infant asthma (typical RR 0.57, 95% CI 0.31 to 1.04; participants = 318; studies = 4), eczema (typical RR 0.93, 95% CI 0.79 to 1.09; participants = 2896; studies = 9), rhinitis (typical RR 0.52, 95% CI 0.14 to 1.85; participants = 256; studies = 3), food allergy (typical RR 1.42, 95% CI 0.87 to 2.33; participants = 479; studies = 2), and CMA (RR 2.31, 95% CI 0.24 to 21.97; participants = 338; studies = 1). Quality of evidence was assessed as very low for all outcomes.
Authors' conclusions: We found no evidence to support short-term or prolonged feeding with a hydrolysed formula compared with exclusive breast feeding for prevention of allergic disease. Very low-quality evidence indicates that short-term use of an EHF compared with a CMF may prevent infant CMA. Further trials are recommended before implementation of this practice.We found no evidence to support prolonged feeding with a hydrolysed formula compared with a CMF for prevention of allergic disease in infants unable to be exclusively breast fed.
Conflict of interest statement
Review authors DO and JS have been invited speakers at industry‐organised scientific meetings. Neither has accepted an honorarium. LJ has no conflicts of interest to declare.
Figures


Update of
-
WITHDRAWN: Infant formulas containing hydrolysed protein for prevention of allergic disease and food allergy.Cochrane Database Syst Rev. 2017 May 25;5(5):CD003664. doi: 10.1002/14651858.CD003664.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Oct 19;10:CD003664. doi: 10.1002/14651858.CD003664.pub6. PMID: 28542713 Free PMC article. Updated.
Comment in
-
Commentary on "Infant Formulas Containing Hydrolysed Protein for Prevention of Allergic Disease and Food Allergy".Neonatology. 2019;116(3):286-289. doi: 10.1159/000495316. Epub 2019 May 23. Neonatology. 2019. PMID: 31121598 No abstract available.
References
References to studies included in this review
Chirico 1997 {published data only}
-
- Chirico G, Gasparoni A, Ciardelli L, Amici M, Colombo A, Rondini G. Immunogenicity and antigenicity of a partially hydrolyzed cow's milk infant formula. Allergy 1997;52(1):82‐8. [PUBMED: 9062633] - PubMed
de Seta 1994 {published data only}
-
- Seta L, Siani P, Cirillo G, Gruttola M, Cimaduomo L, Coletta S. The prevention of allergic diseases with a hypoallergenic formula: a follow‐up at 24 months. The preliminary results. [Italian]. La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics 1994;16(3):251‐4. [PUBMED: 7971447] - PubMed
Halken 2000 {published data only}
-
- Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatric Allergy and Immunology 2000;11(3):149‐61. [PUBMED: 10981524] - PubMed
Hill 2000 {published and unpublished data}
-
- Hill D. A report on the analysis of the Melbourne Atopy Cohort Study. A study designed to test the effectiveness of different formula types on the development of atopic symptoms and signs on a cohort of atopy‐at‐risk infants. Report at year 2. Nestec Internal Report.
-
- Lowe A, Hosking C, Bennett C, Allen K, Carlin J, Dharmage S, et al. The Melbourne Atopy Cohort Study: a RCT of a partially hydrolysed whey formula for the prevention of allergic disease. Internal Medicine Journal 2008;38:A158.
-
- Lowe AJ, Abramson MJ, Hosking CS, Carlin JB, Bennett CM, Dharmage SC, et al. The temporal sequence of allergic sensitization and onset of infantile eczema. Clinical & Experimental Allergy 2007;37(4):536‐42; Erratum in: Clinical & Experimental Allergy 2007; 37(12);1895. [DOI: 10.1111/j.1365-2222.2007.02691.x; PUBMED: 17430350] - DOI - PubMed
Juvonen 1996 {published data only}
-
- Juvonen P, Mansson M, Andersson C, Jakobsson I. Allergy development and macromolecular absorption in infants with different feeding regimens during the first three days of life. A three‐year prospective follow‐up. Acta Paediatrica 1996;85(9):1047‐52. [PUBMED: 8888916] - PubMed
-
- Juvonen P, Mansson M, Jakobsson I. Does early diet have an effect on subsequent macromolecular absorption and serum IgE?. Journal of Pediatric Gastroenterology and Nutrition 1994;18(3):344‐9. [PUBMED: 8057219] - PubMed
-
- Juvonen P, Mansson M, Kjellman NI, Bjorksten B, Jakobsson I. Development of immunoglobulin G and immunoglobulin E antibodies to cow's milk proteins and ovalbumin after a temporary neonatal exposure to hydrolyzed and whole cow's milk proteins. Pediatric Allergy and Immunology 1999;10(3):191‐8. [PUBMED: 10565560] - PubMed
Kwinta 2009 {published data only}
-
- Kwinta P, Sawiec P, Klimek M, Lis G, Cichocka‐Jarosz E, Pietrzyk JJ. Correlation between early neonatal diet and atopic symptoms up to 5‐7 years of age in very low birth weight infants: follow‐up of randomized, double‐blind study. Pediatric Allergy and Immunology 2009;20(5):458‐66. [DOI: 10.1111/j.1399-3038.2008.00814.x; PUBMED: 19490477] - DOI - PubMed
Lam 1992 {unpublished data only}
-
- Lam BC, Yeung CY. The effect of breast milk, infant formula and hypoallergenic formula on incidence of atopic manifestation in high risk infants. Nestle Internal Report 1992.
Mallet 1992 {published data only}
-
- Mallet E, Henocq A. Long‐term prevention of allergic diseases by using protein hydrolysate formula in at‐risk infants. Journal of Pediatrics 1992;121(5 Pt 2):S95‐100. [PUBMED: 1447641] - PubMed
Marini 1996 {published data only}
-
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years' follow‐up. Acta Paediatrica Supplement 1996;414:1‐21. [PUBMED: 8831855] - PubMed
Nentwich 2001 {published data only}
-
- Nentwich I, Michkova E, Nevoral J, Urbanek R, Szepfalusi Z. Cow's milk‐specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy 2001;56(12):1144‐56. [PUBMED: 11736743] - PubMed
Oldaeus 1997 {published data only}
-
- Oldaeus G, Bjorksten B, Jenmalm MC, Kjellman NI. Cow's milk IgE and IgG antibody responses to cow's milk formulas. Allergy 1999;54(4):352‐7. [PUBMED: 10371094] - PubMed
Saarinen 1999 {published data only}
-
- Saarinen KM, Juntunen‐Backman K, Jarvenpaa AL, Kuitunen P, Lope L, Renlund M, et al. Supplementary feeding in maternity hospitals and the risk of cow's milk allergy: a prospective study of 6209 infants. Journal of Allergy and Clinical Immunology 1999;104(2 Pt 1):457‐61. [PUBMED: 10452771] - PubMed
-
- Saarinen KM, Savilahti E. Infant feeding patterns affect the subsequent immunological features in cow's milk allergy. Clinical and Experimental Allergy 2000;30(3):400‐6. [DOI: ] - PubMed
-
- Saarinen KM, Suomalainen H, Savilahti E. Diagnostic value of skin‐prick and patch tests and serum eosinophil cationic protein and cow's milk‐specific IgE in infants with cow's milk allergy. Clinical and Experimental Allergy 2001;31(3):423‐9. [PUBMED: 11260154] - PubMed
-
- Saarinen KM, Vaarala O, Klemetti P, Savilahti E. Transforming growth factor‐beta1 in mothers' colostrum and immune responses to cows' milk proteins in infants with cows' milk allergy. Journal of Allergy and Clinical Immunology 1999;104(5):1093‐8. [PUBMED: 10550758] - PubMed
Tsai 1991 {published data only}
-
- Tsai YT, Chou CC, Hsieh KH. The effect of hypoallergenic formula on the occurrence of allergic diseases in high risk infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1991;32(3):137‐44. [PUBMED: 1776437] - PubMed
Vandenplas 1992 {published data only}
-
- Vandenplas Y. Atopy at 3 years in high‐risk infants fed whey hydrolysate or conventional formula. Lancet 1992;339(8801):1118. [PUBMED: 1349137] - PubMed
-
- Vandenplas Y, Hauser B, Borre C, Clybouw C, Mahler T, Hachimi‐Idrissi S, et al. The long‐term effect of a partial whey hydrolysate formula on the prophylaxis of atopic disease. European Journal of Pediatrics 1995;154(6):488‐94. [PUBMED: 7671948] - PubMed
-
- Vandenplas Y, Hauser B, Borre C, Sacre L, Dab I. Effect of a whey hydrolysate prophylaxis of atopic disease. Annals of Allergy 1992;68(5):419‐24. [PUBMED: 1586005] - PubMed
von Berg 2003 {published and unpublished data}
-
- Berg A, Kramer U, Link E, Bollrath C, Heinrich J, Brockow I, et al. GINIplus study group. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course ‐ the GINIplus study up to the age of 6 years. Clinical and Experimental Allergy 2010;40(4):627‐36. [DOI: 10.1111/j.1365-2222.2009.03444.x; PUBMED: 20082618] - DOI - PubMed
-
- Brockow I, Zutavern A, Hoffmann U, Grubl A, Berg A, Koletzko S, et al. GINIplus Study Group. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. Journal of Investigational Allergology & Clinical Immunology 2009;19(3):180‐7. [DOI: ] - PubMed
-
- Laubereau B, Brockow I, Zirngibl A, Koletzko S, Gruebl A, Berg A, et al. GINI Study Group. Effect of breast‐feeding on the development of atopic dermatitis during the first 3 years of life ‐ results from the GINI‐birth cohort study. Journal of Pediatrics 2004;144(5):602‐7. [DOI: 10.1016/j.jpeds.2003.12.029; PUBMED: 15126993] - DOI - PubMed
-
- Laubereau B, Filipiak‐Pittroff B, Berg A, Grubl A, Reinhardt D, Wichmann HE, et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Archives of Disease in Childhood 2004;89(11):993‐7. [DOI: 10.1136/adc.2003.043265; PUBMED: 15499049] - DOI - PMC - PubMed
-
- Rzehak P, Sausenthaler S, Koletzko S, Bauer CP, Schaaf B, Berg A, et al. Period‐specific growth, overweight and modification by breastfeeding in the GINI and LISA birth cohorts up to age 6 years. European Journal of Epidemiology 2009;24(8):449‐67. [DOI: 10.1007/s10654-009-9356-5; PUBMED: 19521784] - DOI - PubMed
Willems 1993 {published data only}
-
- Willems R, Duchateau J, Magrez P, Denis R, Casimir G. Influence of hypoallergenic milk formula on the incidence of early allergic manifestations in infants predisposed to atopic diseases. Annals of Allergy 1993;71(2):147‐50. [PUBMED: 8346868] - PubMed
References to studies excluded from this review
Agosti 2003 {published data only}
-
- Agosti M, Pugni L, Ramenghi LA, Mosca F, Marini A. Hydrolysed proteins in preterm formula: influence on plasma aminoacids, blood fatty acids and insulinaemia. Acta Paediatrica Supplement 2003;91(441):34‐8. [14599039] - PubMed
Akerblom 2005 {published data only}
-
- Akerblom HK, Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, et al. National TRIGR Study Groups. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: A pilot study. Diabetologia 2005;48(5):829‐37; Erratum in: Diabetologia 2005; Aug;48(8):1676. [DOI: 10.1007/s00125-005-1733-3; PUBMED: 15838685] - DOI - PubMed
-
- Virtanen SM, Barlund S, Salonen M, Savilahti E, Reunanen A, Paronen J, et al. Finnish TRIGR Study Group. Feasibility and compliance in a nutritional primary prevention trial in infants at increased risk for type 1 diabetes. Acta Paediatrica 2011;100(4):557‐64. [DOI: 10.1111/j.1651-2227.2010.02107.x; PUBMED: 21114527] - DOI - PMC - PubMed
Akimoto 1997 {published data only}
-
- Akimoto K, Saito H, Akasawa A, Iikura Y. [Preventative effect of a whey hydrolyzed formula (Nestle, NAN H.A.) on the development of allergic symptoms in infants]. Arerugi [Allergy] 1997;46(10):1044‐51. [PUBMED: 9404092] - PubMed
Arikan 2008 {published data only}
-
- Arikan D, Alp H, Gozum S, Orbak Z, Cifci EK. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing 2008;17(13):1754‐61. [PUBMED: 18592627] - PubMed
Arshad 1990 {unpublished data only}
-
- Arshad SH. Randomised controlled trial of cow’s milk formula versus hydrolysed formula for the prevention of allergy. Author email communication November 2017.
Arshad 1992a {published data only}
-
- Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 1992;339(8808):1493‐7. [PUBMED: 1351183] - PubMed
-
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. Journal of Allergy and Clinical Immunology 1994;93(5):842‐6. [PUBMED: 8182225] - PubMed
-
- Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy 1996;51(2):89‐93. [PUBMED: 8738513] - PubMed
-
- Scott M, Kurukulaaratchy R, Roberts G, Clayton B, Matthews S, Arshad S. Primary prevention of asthma, outcomes at 17 year follow up. Allergy 2010;65(S92):315.
Baldassarre 2017 {published data only}
-
- Baldassarre ME, Mauro A, Fanelli M, Montagna O, Wampler J, Cooper T, et al. Shorter time to full enteral feedings among infants fed an intact protein (IP) vs an extensively hydrolyzed (EH) formula does not appear to be related to differences in gastric emptying. Journal of Pediatric Gastroenterology and Nutrition 2017;64(Suppl 1):920‐1.
Barberi 1993 {unpublished data only}
-
- Barberi I, Salpietro DC, Catalioto G, Fulia F. Clinical trial of a new hypoallergenic formula for risk infants. 2nd World Congress of Perinatal Medicine; 1993 Sep 19‐24; Rome (Italy). 1993.
Bergmann 1996a {published data only}
-
- Bergmann RL, Dannemann A, Edenharter G, Bergmann KE, Monch E, Dudenhausen JW, et al. Influence of ultrafiltrated, partially hydrolysed formula on growth and health of young infants. Monatsschrift fur Kinderheilkunde 1996;144(2):152‐8.
Berseth 2009 {published data only}
Borschel 2013 {published data only}
-
- Borschel MW, Ziegler EE, Wedig RT, Oliver JS. Growth of healthy term infants fed an extensively hydrolyzed casein‐based or free amino acid‐based infant formula: a randomized, double‐blind, controlled trial. Clinical Pediatrics 2013;52(10):910‐7. [DOI: 10.1177/0009922813492883; PUBMED: 23820000] - DOI - PubMed
Borschel 2014a {published data only}
Borschel 2014b {published data only}
-
- Borschel MW, Baggs GE, Barrett‐Reis B. Growth of healthy term infants fed ready‐to‐feed and powdered forms of an extensively hydrolyzed casein‐based infant formula: a randomized, blinded, controlled trial. Clinical Pediatrics 2014;53(6):585‐92. [DOI: 10.1177/0009922814528036; PUBMED: 24662422] - DOI - PubMed
Boyle 2016 {published data only}
-
- Boyle RJ, Tang MLK, Chiang WC, Chua MC, Ismail I, Nauta A, et al. PATCH study investigators. Prebiotic‐supplemented partially hydrolysed cow's milk formula for the prevention of eczema in high‐risk infants: a randomized controlled trial. Allergy 2016;71(5):701‐10. [DOI: 10.1111/all.12848; PUBMED: 27111273] - DOI - PMC - PubMed
-
- Tang M, Rijnierse A, Nauta A, Boyle R, Hourihane J, Chiang W, et al. Influence of early feeding patterns on eczema development in high‐risk infants. Allergy 2017;72:15.
Burks 2008 {published data only}
-
- Burks W, Jones SM, Berseth CL, Harris C, Sampson HA, Scalabrin DM. Hypoallergenicity and effects on growth and tolerance of a new amino acid‐based formula with docosahexaenoic acid and arachidonic acid. Journal of Pediatrics 2008;153(2):266‐71. [DOI: 10.1016/j.jpeds.2008.02.043; PUBMED: 18534230] - DOI - PubMed
Campbell 1989 {published data only}
-
- Campbell JP. Dietary therapy of infant colic: a double‐blind study. Ceskoslovenska Pediatrie 1993;48(4):199‐202. [PUBMED: 8495532] - PubMed
Chan 2002 {published data only}
-
- Chan YH, Shek LP, Aw M, Quak SH, Lee BW. Use of hypoallergenic formula in the prevention of atopic disease among Asian children. Journal of Paediatrics and Child Health 2002;38(1):84‐8. [PUBMED: 11869407] - PubMed
Chandra 1989a {published data only}
Chandra 1989b {published data only}
-
- Chandra RK. Five‐year follow‐up of high‐risk infants with family history of allergy who were exclusively breast‐fed or fed partial whey hydrolysate, soy, and conventional cow's milk formulas. Journal of Pediatric Gastroenterology and Nutrition 1997;24(4):380‐8; Expression of concern in: Journal of Pediatric Gastroenterology and Nutrition 2016; 63(2):307. [PUBMED: 9144119] - PubMed
-
- Chandra RK, Hamed A. Cumulative incidence of atopic disorders in high risk infants fed whey hydrolysate, soy, and conventional cow milk formulas. Annals of Allergy 1991;67(2 Pt 1):129‐32. [PUBMED: 1867449] - PubMed
-
- Chandra RK, Singh G, Shridhara B. Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Annals of Allergy 1989;63(2):102‐6. [PUBMED: 2669565] - PubMed
Chan‐Yeung 2000 {published data only}
-
- Chan‐Yeung M, Manfreda J, Dimich‐Ward H, Ferguson A, Watson W, Becker A. A randomized controlled study on the effectiveness of a multifaceted intervention program in the primary prevention of asthma in high‐risk infants. Archives of Pediatrics & Adolescent Medicine 2000;154(7):657‐63. [PUBMED: 10891016] - PubMed
Corvaglia 2013 {published data only}
D'Agata 1996 {published data only}
-
- D'Agata A, Betta P, Sciacca P, Morano C, Pratico G, Curreri R, et al. Role of dietary prevention in newborns at risk for atopy. Results of a follow‐up study. Pediatria Medica e Chirurgica 1996;18(5):469‐72. [PUBMED: 9053884] - PubMed
Decsi 1992 {published data only}
-
- Decsi T, Volker V, Szasz M, Ezer E, Mehes K. [Comparison of human milk with 3 different types of infant food in the nutrition of full‐term neonates]. Orvosi Hetilap 1992;133(33):2087‐91. [PUBMED: 1501859] - PubMed
Decsi 1998 {published data only}
-
- Decsi T, Veitl V, Burus I. Plasma amino acid concentrations, indexes of protein metabolism and growth in healthy, full‐term infants fed partially hydrolyzed infant formula. Journal of Pediatric Gastroenterology and Nutrition 1998;27(1):12‐6. [PUBMED: 9669720] - PubMed
de Jong 1998 {published data only}
Exl 1998 {published data only}
-
- Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen reduced dietary intervention programme. The ZUFF‐study‐programme. Part I: study design and 6‐month nutritional behaviour. European Journal of Nutrition 2000;39(3):89‐102. [PUBMED: 10918990] - PubMed
-
- Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen‐reduced dietary intervention programme: the ZUFF‐STUDY‐PROGRAMME. Part II: infant growth and health status to age 6 months. European Journal of Nutrition 2000;39(4):145‐56. [PUBMED: 11079734] - PubMed
-
- Exl BM, Deland U, Wall M, Preysch U, Secretin MC, Shmerling DH. Zug‐Frauenfeld Nutritional Survey ('Zuff Study'): allergen‐reduced nutrition in a normal infant population and its health‐related effects. Results at the age of six months. Nutrition Research 1998;18(8):1443‐62. [DOI: 10.1016/S0271-5317(98)00121-3] - DOI
Florendo 2009 {published data only}
Fukushima 1997 {published data only}
-
- Fukushima Y, Iwamoto K, Takeuchi‐Nakashima A, Akamatsu N, Fujino‐Numata N, Yoshikoshi M, et al. Preventive effect of whey hydrolysate formulas for mothers and infants against allergy development in infants for the first 2 years. Journal of Nutritional Science and Vitaminology 1997;43(3):397‐411. [PUBMED: 9268927] - PubMed
Giovannini 1994 {published data only}
-
- Giovannini M, Agostoni C, Fiocchi A, Bellu R, Trojan S, Riva E. Antigen‐reduced infant formulas versus human milk: growth and metabolic parameters in the first 6 months of life. Journal of the American College of Nutrition 1994;13(4):357‐63. [PUBMED: 7963141] - PubMed
Halken 1992 {published data only}
-
- Halken S, Host A, Hansen LG, Osterballe O. Effect of an allergy prevention programme on incidence of atopic symptoms in infancy. A prospective study of 159 "high‐risk" infants. Allergy 1992;47(5):545‐53. [PUBMED: 1485660] - PubMed
-
- Halken S, Host A, Hansen LG, Osterballe O. Prevention of allergy in infants. A prospective study of 159 high‐risk children. Ugeskrift for Laeger 1994;156(3):308‐12. [PUBMED: 8296423] - PubMed
-
- Halken S, Host A, Hansen LG, Osterballe O. Preventive effect of feeding high‐risk infants a casein hydrolysate formula or an ultrafiltrated whey hydrolysate formula. A prospective, randomized, comparative clinical study. Pediatric Allergy and Immunology 1993;4(4):173‐81. [PUBMED: 8298708] - PubMed
Hartman 1994 {unpublished data only}
-
- Hartman CT, Fredericks GL, Katz ES, Brown CA. Prevalence of symptomatic formula intolerance and allergy in a general infant population. Journal of Allergy and Clinical Immunology 1994;93(1):210. [(A286)]
Hattevig 1989 {published data only}
-
- Hattevig G, Kjellman B, Sigurs N, Bjorksten B, Kjellman NI. Effect of maternal avoidance of eggs, cow's milk and fish during lactation upon allergic manifestations in infants. Clinical and Experimental Allergy 1989;19(1):27‐32. [PUBMED: 2702510] - PubMed
-
- Hattevig G, Kjellman B, Sigurs N, Grodzinsky E, Hed J, Bjorksten B. The effect of maternal avoidance of eggs, cow's milk, and fish during lactation on the development of IgE, IgG, and IgA antibodies in infants. Journal of Allergy and Clinical Immunology 1990;85(1 Pt 1):108‐15. [PUBMED: 2299096] - PubMed
-
- Hattevig G, Sigurs N, Kjellman B. Effects of maternal dietary avoidance during lactation on allergy in children at 10 years of age. Acta Paediatrica 1999;88(1):7‐12. [PUBMED: 10090539] - PubMed
-
- Paronen J, Bjorksten B, Hattevig G, Akerblom HK, Vaarala O. Effect of maternal diet during lactation on development of bovine insulin‐binding antibodies in children at risk for allergy. Journal of Allergy and Clinical Immunology 2000;106(2):302‐6. [DOI: 10.1067/mai.2000.108110; PUBMED: 10932074] - DOI - PubMed
-
- Sigurs N, Hattevig G, Kjellman B. Maternal avoidance of eggs, cow's milk, and fish during lactation: effect on allergic manifestations, skin‐prick tests, and specific IgE antibodies in children at age 4 years. Pediatrics 1992;89(4 Pt 2):735‐9. [PUBMED: 1557270] - PubMed
Hernell 2003 {published data only}
Hill 1995b {published data only}
-
- Hill DJ, Hudson IL, Sheffield LJ, Shelton MJ, Menahem S, Hosking CS. A low allergen diet is a significant intervention in infantile colic: results of a community‐based study. Journal of Allergy and Clinical Immunology 1995;96(6 Pt 1):886‐92. [PUBMED: 8543745] - PubMed
Høst 1991 {published data only}
-
- Høst A. Importance of the first meal on the development of cow's milk allergy and intolerance. Allergy Proceedings 1991;12(4):227‐32. [PUBMED: 1936970] - PubMed
Iikura 1995 {unpublished data only}
-
- Iikura Y, Akimoto K, Ebisawa M, Onda T, Akazawa A, Saito H, et al. Effect of hydrolyzed whey protein formula for babies on development of allergic symptoms during infancy. Nestle Nutrition Workshop Series. Vol. 34, Baltimore, Maryland: Raven Press, 1995:269‐75.
Keller 1996 {published data only}
-
- Keller KM, Burgin‐Wolff A, Lippold R, Wirth S, Lentze MJ. The diagnostic significance of IgG cow's milk protein antibodies re‐evaluated. European Journal of Pediatrics 1996;155(4):331‐7. [PUBMED: 8777930] - PubMed
Knip 2010 {published data only}
-
- Hyytinen M, Savilahti E, Virtanen SM, Harkonen T, Ilonen J, Luopajarvi K, et al. Finnish TRIGR Pilot Study Group. Avoidance of cow's milk‐based formula for at‐risk infants does not reduce development of celiac disease: a randomized controlled trial. Gastroenterology 2017;153(4):961‐70.e3. - PubMed
Knip 2011 {published data only}
-
- Knip M, Virtanen SM, Becker D, Dupre J, Krischer JP, Akerblom HK, TRIGR Study Group. Early feeding and risk of type 1 diabetes: experiences from the Trial to Reduce Insulin‐dependent diabetes mellitus in the Genetically at Risk (TRIGR). American Journal of Clinical Nutrition 2011;94(6 Suppl):1814S‐20S. [DOI: 10.3945/ajcn.110.000711; PUBMED: 21653795] - DOI - PMC - PubMed
-
- NCT00179777. TRIGR ‐ Primary prevention study for Type 1 Diabetes in children at risk [TRIGR ‐ Trial to Reduce IDDM in the Genetically at Risk]. clinicaltrials.gov/show/NCT00179777 (first received 16 September 2005).
-
- TRIGR Study Group, Akerblom HK, Krischer J, Virtanen SM, Berseth C, Becker D, Dupre J, et al. The trial to reduce IDDM in the genetically at risk (TRIGR) study: recruitment, intervention and follow‐up. Diabetologia 2011;54(3):627‐33; Erratum in: Diabetologia. 2011;54(8):2210. [DOI: 10.1007/s00125-010-1964-9; NCT00179777; PUBMED: 21153533] - DOI - PMC - PubMed
Kuo 2011 {published data only}
-
- Kuo HC, Liu CA, Ou CY, Hsu TY, Wang CL, Huang HC, et al. Partial protein‐hydrolyzed infant formula decreased food sensitization but not allergic diseases in a prospective birth cohort study. International Archives of Allergy and Immunology 2011;154(4):310‐7. [DOI: 10.1159/000321823; PUBMED: 20975282] - DOI - PubMed
Lasekan 2006 {published data only}
-
- Lasekan JB, Koo WW, Walters J, Neylan M, Luebbers S. Growth, tolerance and biochemical measures in healthy infants fed a partially hydrolyzed rice protein‐based formula: a randomized, blinded, prospective trial. Journal of the American College of Nutrition 2006;25(1):12‐9. [PUBMED: 16522927] - PubMed
Lucassen 2000 {published data only}
-
- Lucassen PL, Assendelft WJ, Gubbels JW, Eijk JT, Douwes AC. Infantile colic: crying time reduction with a whey hydrolysate. A double‐blind, randomized, placebo‐controlled trial. Pediatrics 2000;106(6):1349‐54. [PUBMED: 11099588] - PubMed
Maggio 2005 {published data only}
-
- Maggio L, Zuppa AA, Sawatzki G, Valsasina R, Schubert W, Tortorolo G. Higher urinary excretion of essential amino acids in preterm infants fed protein hydrolysates. Acta Paediatrica 2005;94(1):75‐84. [PUBMED: 15858965] - PubMed
-
- Zuppa AA, Girlando P, Scapillati ME, Maggio L, Romagnoli C, Tortorolo G. [Effects on growth, tolerability and biochemical parameters of two different human milk fortifiers in very low birth‐weight newborns]. Pediatria Medica e Chirurgica 2004;26(1):45‐9. [PUBMED: 15529811] - PubMed
Martinez‐Valverde 1993 {unpublished data only}
-
- Martinenez‐Valverde A, Aljma‐Garcia JM. Study and evaluation of serum IgE and specific total IgE against alpha‐lactalbumin, beta‐lactoglobulin and casein with hypoallergenic formulas (HA) and other types of feeding [Thesis]. Spain: Universidad de Malaga, 1993.
Medjad‐Guillou 1992 {published data only}
-
- Medjad‐Guillou N, Henocq A, Arnaud‐Battandier F. Does the hydrolysis of proteins change the acceptability and the digestive tolerance of milk for infants? The results of a comparative and randomized prospective study. Annales de Pediatrie 1992;39(3):202‐6. [PUBMED: 1570949] - PubMed
Mennella 2011a {published data only}
Mihatsch 1999 {published data only}
-
- Mihatsch WA, Hogel J, Pohlandt F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatrica 2001;90(2):196‐8. [PUBMED: 11236051] - PubMed
-
- Mihatsch WA, Pohlandt F. Protein hydrolysate formula maintains homeostasis of plasma amino acids in preterm infants. Journal of Pediatric Gastroenterology and Nutrition 1999;29(4):406‐10. [PUBMED: 10512399] - PubMed
Mihatsch 2002 {published data only}
-
- Mihatsch WA, Franz AR, Hogel J, Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002;110(6):1199‐203. [PUBMED: 12456919] - PubMed
Mitchell 1977 {published data only}
-
- Mitchell JD, Brand J, Halbisch J. Weight‐gain inhibition by lactose in Australian Aboriginal children. A controlled trial of normal and lactose hydrolysed milk. Lancet 1977;1(8010):500‐2. [PUBMED: 65606] - PubMed
Moran 1992 {published data only}
-
- Moran JR. Effects of prolonged exposure to partially hydrolyzed milk protein. Journal of Pediatrics 1992;121(5 Pt 2):S90‐4. [PUBMED: 1447640] - PubMed
NCT00936637 {published data only}
-
- NCT00936637. Effects on growth of an extensively hydrolyzed formula fed to term [A 16‐week growth study of an extensively hydrolyzed infant formula, 3 Months treatment and 1 month follow‐up for a duration of 4 months]. clinicaltrials.gov/show/NCT00936637 (first received 10 July 2009).
NCT01987154 {published data only}
-
- NCT01987154. Tolerance of an extensively hydrolyzed protein infant formula versus a premature infant formula. clinicaltrials.gov/show/NCT01987154 (first received 19 November 2013).
Nentwich 2003 {published data only}
-
- Nentwich I, Pazdirkova A, Koberska I, Pokojska E, Szepfalusi Z, Lokaj J. Cow milk‐specific humoral and cellular immune response in infants with high risk of atopy under feeding a whey hydrolysate infant formula. Klinische Padiatrie 2003;215(5):275‐9. [DOI: 10.1055/s-2003-42664; PUBMED: 14520590] - DOI - PubMed
Nocerino 2012 {published data only}
-
- Nocerino R, Marco G, Cosenza L, Pezzella V, Passariello A, Terrin G, et al. Efficacy of a new partially hydrolyzed whey formula on infant colic: a randomized controlled trial. Digestive and Liver Disease 2012;44:S272.
Odelram 1996 {published data only}
-
- Odelram H, Vanto T, Jacobsen L, Kjellman NI. Whey hydrolysate compared with cow's milk‐based formula for weaning at about 6 months of age in high allergy‐risk infants: effects on atopic disease and sensitization. Allergy 1996;51(3):192‐5. [PUBMED: 8781676] - PubMed
Paronen 2000 {published data only}
-
- Paronen J, Knip M, Savilahti E, Virtanen SM, Ilonen J, Akerblom HK, et al. Effect of cow's milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Finnish Trial to Reduce IDDM in the Genetically at Risk Study Group. Diabetes 2000;49(10):1657‐65. [PUBMED: 11016449] - PubMed
Pauls 1996 {published data only}
-
- Pauls J, Bauer K, Versmold H. Randomized controlled trial of formulas with hydrolyzed versus non‐hydrolyzed protein for starting enteral feedings in preterm infants <1500g body weight. Journal of Pediatric Gastroenterology and Nutrition 1996;22:450 (abstract 164).
Picaud 2001 {published data only}
-
- Picaud JC, Lapillonne A, Rigo J, Normand S, Reygrobellet B, Claris O, et al. Nitrogen utilization and bone mineralization in very low birth weight infants fed partially hydrolyzed preterm formula. Seminars in Perinatology 2002;26(6):439‐46; Retraction in: Seminars in Perinatology 2013; 37(2):138. [PUBMED: 12537316] - PubMed
-
- Picaud JC, Rigo J, Normand S, Lapillonne A, Reygrobellet B, Claris O, et al. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: a randomized pilot study. Journal of Pediatric Gastroenterology and Nutrition 2001;32(5):555‐61. [PUBMED: 11429516] - PubMed
Porch 1998 {published data only}
-
- Porch MC, Shahane AD, Leiva LE, Elston RC, Sorensen RU. Influence of breast milk, soy or two hydrolyzed formulas on the development of allergic manifestations in infants at risk. Nutrition Research 1998;18(8):1413‐24.
Raupp 1995 {published data only}
-
- Raupp P, Kries R, Gobel C, Fox A, Lemburg P, Schmidt E, et al. Bone density and biochemical parameters of bone mineral metabolism and acid load in preterm infants. Cow's milk formula versus hydrolysed protein preparation. Monatsschr Kinderheilkd 1995;143(7 (Suppl 2)):S140‐6.
Riezzo 2001 {published data only}
-
- Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, et al. Gastric electrical activity and gastric emptying in preterm newborns fed standard and hydrolysate formulas. Journal of Pediatric Gastroenterology and Nutrition 2001;33(3):290‐5. [PUBMED: 11593124] - PubMed
Rigo 1994a {published data only}
-
- Rigo J, Salle BL, Picaud JC, Putet G, Senterre J. Nutritional evaluation of protein hydrolysate formulas. European Journal of Clinical Nutrition 1995;49(Suppl 1):S26‐38. [PUBMED: 8647061] - PubMed
-
- Rigo J, Salle BL, Putet G, Senterre J. Nutritional evaluation of various protein hydrolysate formulae in term infants during the first month of life. Acta Paediatrica Supplement 1994;402:100‐4. [PUBMED: 7841611] - PubMed
Rigo 1994b {published data only}
-
- Rigo J, Senterre J. Metabolic balance studies and plasma amino acid concentrations in preterm infants fed experimental protein hydrolysate preterm formulas. Acta Paediatrica Supplement 1994;405:98‐104. [PUBMED: 7734800] - PubMed
Savino 2003 {published data only}
-
- Savino F, Cresi F, Maccario S, Cavallo F, Dalmasso P, Fanaro S, et al. "Minor" feeding problems during the first months of life: effect of a partially hydrolysed milk formula containing fructo‐ and galacto‐oligosaccharides. Acta Paediatrica Supplement 2003;91(441):86‐90. [PUBMED: 14599049] - PubMed
Savino 2006 {published data only}
-
- Savino F, Palumeri E, Castagno E, Cresi F, Dalmasso P, Cavallo F, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. European Journal of Clinical Nutrition 2006;60(11):1304‐10. [DOI: 10.1038/sj.ejcn.1602457; PUBMED: 16736065] - DOI - PubMed
Scalabrin 2009 {published data only}
-
- Scalabrin D, Berseth C, Marunycz J, Mitmesser S. Long term safety, growth, and health effects of lactobacillus GG (LGG) added to partially and extensively hydrolyzed infant formulas. Allergy: European Journal of Allergy and Clinical Immunology 2009;64(1171):447.
-
- Scalabrin D, Harris C, Johnston WH, Berseth CL. Long‐term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: a 5‐year follow‐up. European Journal of Pediatrics 2017;176(2):217‐24. [DOI: 10.1007/s00431-016-2825-4; PUBMED: 27975116] - DOI - PMC - PubMed
-
- Scalabrin DM, Johnston WH, Hoffman DR, P'Pool VL, Harris CL, Mitmesser SH. Growth and tolerance of healthy term infants receiving hydrolyzed infant formulas supplemented with Lactobacillus rhamnosus GG: randomized, double‐blind, controlled trial. Clinical Pediatrics 2009;48(7):734‐44. [DOI: 10.1177/0009922809332682; NCT00655720; PUBMED: 19264721] - DOI - PubMed
-
- Scalabrin DMF, Harris C, Strong PV, Liu B, Berseth CL. Infant supplementation with Lactobacillus rhamnosus GG (LGG) and long‐term growth and health: a 5‐year follow‐up. Allergy: European Journal of Allergy and Clinical Immunology 2014;69(451):196‐7.
Schmelzle 2003 {published data only}
-
- Schmelzle H, Wirth S, Skopnik H, Radke M, Knol J, Bockler HM, et al. Randomized double‐blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta‐palmitic acid level, and nondigestible oligosaccharides. Journal of Pediatric Gastroenterology and Nutrition 2003;36(3):343‐51. [PUBMED: 12604972] - PubMed
Schmidt 1995 {published data only}
-
- Schmidt E, Eden‐Kohler J, Tonkaboni F, Tolle J. Alimentary allergy prevention in infants with increased allergic risk: the effect of different feeding regimens in the first 6 months on atopic manifestations during the first year of life. A large scale feeding trial. Nestle Nutritional Workshop Series. Vol. 34, Baltimore, Maryland: Raven Press, 1995:231‐48.
Schmitz 1992 {published data only}
-
- Schmitz J, Digeon B, Chastang C, Dupouy D, Leroux B, Robillard P, et al. Effects of brief early exposure to partially hydrolyzed and whole cow milk proteins. Journal of Pediatrics 1992;121(5 Pt 2):S85‐9. [PUBMED: 1447639] - PubMed
Schrander 1993 {published data only}
-
- Schrander JJ, Bogart JP, Forget PP, Schrander‐Stumpel CT, Kuijten RH, Kester AD. Cow's milk protein intolerance in infants under 1 year of age: a prospective epidemiological study. European Journal of Pediatrics 1993;152(8):640‐4. [PUBMED: 8404966] - PubMed
Shao 2006 {published data only}
-
- Shao J, Sheng J, Dong W, Li YZ, Yu SC. Effects of feeding intervention on development of eczema in atopy high‐risk infants: an 18‐month follow‐up study. Zhonghua Er Ke Za Zhi 2006;44(9):684‐7. [PUBMED: 17217664] - PubMed
Silva Rey 1996 {unpublished data only}
-
- Silva Rey AL, Garcia G, Nogales A. Preventatibve Effect of a Partially Hydrolyzed Milk Formula [Thesis]. Madrid, Spain, 1996.
Sorensen 2007 {published data only}
-
- Sorensen RU, Inostroza J, Hebal E, Vinet AM, Fierro J. Weaning to a partially hydrolyzed whey formula at three months of age protects against the later development of anti‐cow milk lgE antibodies in comparison to whole cows milk or extensively hydrolyzed whey formulas. Journal of Allergy and Clinical Immunology 2007;119(1):S120.
Staelens 2008 {published data only}
-
- Staelens S, Driessche M, Barclay D, Carrie‐Faessler AL, Haschke F, Verbeke K, et al. Gastric emptying in healthy newborns fed an intact protein formula, a partially and an extensively hydrolysed formula. Clinical Nutrition 2008;27(2):264‐8. [DOI: 10.1016/j.clnu.2007.12.009; PUBMED: 18280619] - DOI - PubMed
Szajewska 2001 {published data only}
-
- Szajewska H, Albrecht P, Stoinska B, Prochowska A, Gawecka A, Laskowska‐Klita T. Extensive and partial protein hydrolysate preterm formulas: the effect on growth rate, protein metabolism indices, and plasma amino acid concentrations. Journal of Pediatric Gastroenterology and Nutrition 2001;32(3):303‐9. [PUBMED: 11345180] - PubMed
Szajewska 2004 {published data only}
-
- Szajewska H, Mrukowicz JZ, Stoinska B, Prochowska A. Extensively and partially hydrolysed preterm formulas in the prevention of allergic diseases in preterm infants: a randomized, double‐blind trial. Acta Paediatrica 2004;93(9):1159‐65. [PUBMED: 15384877] - PubMed
Tariq 1998 {published data only}
Taubman 1988 {published data only}
-
- Taubman B. Parental counseling compared with elimination of cow's milk or soy milk protein for the treatment of infant colic syndrome: a randomized trial. Pediatrics 1988;81(6):756‐61. [PUBMED: 3285312] - PubMed
Vaarala 1995 {published data only}
-
- Vaarala O, Saukkonen T, Savilahti E, Klemola T, Akerblom HK. Development of immune response to cow's milk proteins in infants receiving cow's milk or hydrolyzed formula. Journal of Allergy and Clinical Immunology 1995;96(6 Pt 1):917‐23. [PUBMED: 8543750] - PubMed
Vaarala 2012 {published data only}
-
- Vaarala O, Ilonen J, Ruohtula T, Pesola J, Virtanen SM, Harkonen T, et al. Removal of bovine insulin from cow's milk formula and early initiation of beta‐cell autoimmunity in the FINDIA pilot study. Archives of Pediatrics & Adolescent Medicine 2012;166(7):608‐14. [DOI: 10.1001/archpediatrics.2011.1559; PUBMED: 22393174] - DOI - PubMed
Vandenplas 1988 {published data only}
-
- Vandenplas Y, Deneyer M, Sacre L, Loeb H. Preliminary data on a field study with a new hypo‐allergenic formula. European Journal of Pediatrics 1988;148(3):274‐7. [PUBMED: 3215202] - PubMed
-
- Vandenplas Y, Malfroot A, Dab I. Short‐term prevention of cow's milk protein allergy in infants. Immunology and Allergy Practice 1989;11:430‐7.
Vandenplas 1993 {published data only}
-
- Hauser B, Blecker U, Keymolen K, Suys B, Gerlo E, Vandenplas Y. Plasma amino acid concentrations in term‐born infants fed a whey predominant or a whey hydrolysate formula. JPEN. Journal of Parenteral and Enteral Nutrition 1997;21(1):27‐30. [DOI: 10.1177/014860719702100127; PUBMED: 9002081] - DOI - PubMed
-
- Hauser B, Keymolen K, Blecker U, Suys B, Bougatef A, Loeb H, et al. A comparative evaluation of whey hydrolysate and whey‐predominant formulas. How well do infants accept and tolerate them?. Clinical Pediatrics 1993;62:433‐7. - PubMed
-
- Vandenplas Y, Hauser B, Blecker U, Suys B, Peeters S, Keymolen K, et al. The nutritional value of a whey hydrolysate formula compared with a whey‐predominant formula in healthy infants. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):92‐6. [PUBMED: 8350218] - PubMed
Wopereis 2014 {published data only}
-
- Wopereis H, Sim K, Shaw A, Martin R, Oozeer R, Warner JO, et al. The developmental gut microbiota is modulated towards a pattern closer to breastfed infants by a partially hydrolyzed cow's milk formula supplemented with specific prebiotic oligosaccharides. Allergy: European Journal of Allergy and Clinical Immunology 2014;69:315.
Xinias 2017 {published data only}
-
- Xinias I, Analitis A, Vandenplas Y. A synbiotic partial whey hydrolysate with reduced lactose decreases infantile colic and improves quality of life; A randomized open pilot study. Journal of Pediatric Gastroenterology and Nutrition 2017;64:143.
Yu 2014 {published data only}
-
- Yu MX, Zhuang SQ, Wang DH, Zhou XY, Liu XH, Shi LP, et al. Effects of extensively hydrolyzed protein formula on feeding and growth in preterm infants: a multicenter controlled clinical study. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2014;16(7):684‐90. [PUBMED: 25008873] - PubMed
Zeiger 1989 {published data only}
-
- Zeiger RS, Heller S. Development of nasal basophilic cells and nasal eosinophils from age 4 months through 4 years in children of atopic parents. Journal of Allergy and Clinical Immunology 1993;91(3):723‐34. [PUBMED: 8454794] - PubMed
-
- Zeiger RS, Heller S. The development and prediction of atopy in high‐risk children: follow‐up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. Journal of Allergy and Clinical Immunology 1995;95(6):1179‐90. [PUBMED: 7797786] - PubMed
-
- Zeiger RS, Heller S, Mellon MH, Forsythe AB, O'Connor RD, Hamburger RN, et al. Effect of combined maternal and infant food‐allergen avoidance on development of atopy in early infancy: a randomized study. Journal of Allergy and Clinical Immunology 1989;84:72‐89. - PubMed
-
- Zeiger RS, Heller S, Mellon MH, Halsey JF, Hamburger RN, Sampson HA. Genetic and environmental factors affecting the development of atopy through age 4 in children of atopic parents: a prospective randomized study of food allergen avoidance. Pediatric Allergy and Immunology 1992;3(3):110‐27.
References to ongoing studies
NCT01036243 {published data only}
-
- NCT01036243. Digestive tolerance of slightly hydrolyzed starter infant formula with probiotics [Tolerance of a slightly hydrolyzed starter formula containing probiotics]. clinicaltrials.gov/show/NCT01036243 (first received 21 December 2009).
NCT01156493 {published data only}
-
- NCT01156493. Hydrolized protein formula for premature infants [Randomized trial of hydrolyzed protein premature formula]. clinicaltrials.gov/show/ NCT01156493 (first received 02 July 2010).
NCT01210391 {unpublished data only}
-
- NCT01210391. Growth of infants fed an extensively hydrolyzed infant formula [Assessment of growth of infants fed a 100% whey extensively hydrolyzed formula]. clinicaltrials.gov/show/NCT01210391 (first received 28 September 2010).
NCT01700205 {published data only}
-
- NCT01700205. "Watch your baby grow" study (GRO) [Impact of diet composition on energy balance and satiety during infancy]. clinicaltrials.gov/show/NCT01700205 (first received 4 October 2012).
NCT01735123 {unpublished data only}
-
- NCT01735123. Early dietary intervention and later signs of beta‐cell autoimmunity (EDIA). clinicaltrials.gov/show/NCT01735123 (first received 28 November 2012).
Yin 2015 {published data only}
-
- Yin LP, Qian LJ, Zhu H, Chen Y, Li H, Han JN, et al. Application effect of extensively hydrolyzed milk protein formula and follow‐up in preterm children with a gestational age of less than 34 weeks: study protocol for a randomized controlled trial. Trials 2015;16:498. [DOI: 10.1186/s13063-015-1030-5; PUBMED: 26537897] - DOI - PMC - PubMed
Additional references
AAP 2000
-
- Americal Academy of Pediatrics: Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000;106(2 Pt 1):346‐9. [PUBMED: 10920165] - PubMed
Alexander 2010
Allen 2009
Arshad 2005
Bergmann 1994
-
- Bergmann RL, Bergmann KE, Lau‐Schadensdorf S, Luck W, Dannemann A, Bauer CP, et al. Atopic diseases in infancy. The German multicenter atopy study (MAS‐90). Pediatric Allergy and Immunology 1994;5(6 Suppl):19‐25. [PUBMED: 7728224] - PubMed
Bergmann 1997
-
- Bergmann RL, Edenharter G, Bergmann KE, Guggenmoos‐Holzmann I, Forster J, Bauer CP, et al. Predictability of early atopy by cord blood‐IgE and parental history. Clinical and Experimental Allergy 1997;27(7):752‐60. [PUBMED: 9249267] - PubMed
Bergmann 1998
-
- Bergmann R, Woodcock A. Whole population or high‐risk group? Childhood asthma. European Respiratory Journal. Supplement 1998;27:9S‐12S. [PUBMED: 9699777] - PubMed
Bock 1988
-
- Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double‐blind, placebo‐controlled food challenge (DBPCFC) as an office procedure: a manual. Journal of Allergy and Clinical Immunology 1988;82(6):986‐97. [PUBMED: 3060514] - PubMed
Boyle 2016a
Burr 1989
Chaffen 2010
Chung 2012
Custovic 2000
-
- Custovic A, Simpson BM, Simpson A, Hallam C, Craven M, Brutsche M, et al. Manchester Asthma and Allergy Study: low‐allergen environment can be achieved and maintained during pregnancy and in early life. Journal of Allergy and Clinical Immunology 2000;105(2 Pt 1):252‐8. [PUBMED: 10669844] - PubMed
Custovic 2001
-
- Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. NAC Manchester Asthma and Allergy Study Group. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet 2001;358(9277):188‐93. [DOI: 10.1016/S0140-6736(01)05406-X; PUBMED: 11476835] - DOI - PubMed
Darsow 2000
-
- Darsow U, Ring J. Airborne and dietary allergens in atopic eczema: a comprehensive review of diagnostic tests. Clinical and Experimental Dermatology 2000;25(7):544‐51. [PUBMED: 11122226] - PubMed
de Silva 2014
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 22 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gruskay 1982
Halken 2004
Hansen 1993
-
- Hansen LG, Halken S, Host A, Moller K, Osterballe O. Prediction of allergy from family history and cord blood IgE levels. A follow‐up at the age of 5 years. Cord blood IgE. IV. Pediatric Allergy and Immunology 1993;4(1):34‐40. [PUBMED: 8348254] - PubMed
Higgins 2017
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Hill 2007
Høst 1994
-
- Høst A. Cow's milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatric Allergy and Immunology 1994;5(5 Suppl):1‐36. - PubMed
Høst 1995
-
- Høst A, Jacobsen HP, Halken S, Holmenlund D. The natural history of cow's milk protein allergy/intolerance. European Journal of Clinical Nutrition 1995;49(Suppl 1):S13‐8. [PUBMED: 8647059] - PubMed
Høst 1999
-
- Høst A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, et al. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Archives of Disease in Childhood 1999;81(1):80‐4. [PUBMED: 10373144] - PMC - PubMed
Iskedjian 2010
-
- Iskedjian M, Szajewska H, Spieldenner J, Farah B, Berbari J. Meta‐analysis of a partially hydrolysed 100%‐whey infant formula vs. extensively hydrolysed infant formulas in the prevention of atopic dermatitis. Current Medical Research and Opinion 2010;26(11):2599‐606. [DOI: 10.1185/03007995.2010.525475; PUBMED: 20925453] - DOI - PubMed
Johansson 2004
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use. Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy and Clinical Immunology 2004;113(5):832‐6. [DOI: 10.1016/j.jaci.2003.12.591; PUBMED: 15131563] - DOI - PubMed
Katz 2010
-
- Katz Y, Rajuan N, Goldberg MR, Eisenberg E, Heyman E, Cohen A, et al. Early exposure to cow's milk protein is protective against IgE‐mediated cow's milk protein allergy. Journal of Allergy and Clinical Immunology 2010;126(1):77‐82 e1. [DOI: 10.1016/j.jaci.2010.04.020; PUBMED: 20541249] - DOI - PubMed
Katz 2011
-
- Katz Y, Goldberg MR, Rajuan N, Cohen A, Leshno M. The prevalence and natural course of food protein‐induced enterocolitis syndrome to cow's milk: a large‐scale, prospective population‐based study. Journal of Allergy and Clinical Immunology 2011;127(3):647‐53 e1‐3. [DOI: 10.1016/j.jaci.2010.12.1105; PUBMED: 21377033] - DOI - PubMed
Kilpeläinen 2001
-
- Kilpeläinen M, Terho EO, Helenius H, Koskenvuo M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy 2001;56(5):377‐84. [PUBMED: 11350300] - PubMed
Kjellman 1977
-
- Kjellman NI. Atopic disease in seven‐year‐old children. Incidence in relation to family history. Acta Paediatrica Scandinavica 1997;66(4):465‐71. [PUBMED: 899762] - PubMed
Kleinman 1991
-
- Kleinman RE, Bahna S, Powell GF, Sampson HA. Use of infant formulas in infants with cow milk allergy. A review and recommendations. Pediatric Allergy and Immunology 1991;2(4):146‐55.
Kramer 2012
Kwinta 2002
-
- Kwinta P, Mitkowska Z, Kruczek P, Tomasik T, Pietrzyk JJ. Influence of the lactose free and lactose containing diet on prevalence of gram‐negative sepsis and feeding intolerance in very low birth weight infants: double‐blind randomized trial. Przeglad Lekarski 2002;59(Suppl 1):63–6. [PUBMED: 12108078] - PubMed
Muraro 2004
-
- Muraro A, Dreborg S, Halken S, Host A, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Part II. Evaluation of methods in allergy prevention studies and sensitization markers. Definitions and diagnostic criteria of allergic diseases. Pediatric Allergy and Immunology 2004;15(3):196‐205. [DOI: 10.1111/j.1399-3038.2004.00128.x; PUBMED: 15209950] - DOI - PubMed
Muraro 2014
-
- Muraro A, Halken S, Arshad SH, Beyer K, Dubois AE, Du Toit G, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014;69(5):590‐601. - PubMed
Oddy 1999
Osterballe 2005
Peat 2000
-
- Peat JK, Toelle BG, Mellis CM. Problems and possibilities in understanding the natural history of asthma. Journal of Allergy and Clinical Immunology 2000;106(3 Suppl):S144‐52. [PUBMED: 10984395] - PubMed
Peat 2001
Prescott 2005
-
- Prescott SL, Tang ML, Australasian Society of Clinical Immunology and Allergy. The Australasian Society of Clinical Immunology and Allergy position statement: Summary of allergy prevention in children. Medical Journal of Australia 2005;182(9):464‐7. [PUBMED: 15865590] - PubMed
Ravault 2001
-
- Ravault C, Kauffmann F. Validity of the IUATLD (1986) questionnaire in the EGEA study. International Union Against Tuberculosis and Lung Disease. Epidemiological study on the genetics and environment of asthma, bronchial hyperresponsiveness and atopy. Journal of Tuberculosis and Lung Disease 2001;5(2):191‐6. [PUBMED: 11258514] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronmark 2001
-
- Ronmark E, Jonsson E, Platts‐Mills T, Lundback B. Incidence and remission of asthma in schoolchildren: report from the obstructive lung disease in northern Sweden studies. Pediatrics 2001;107(3):E37. [PUBMED: 11230618] - PubMed
Saarinen 1995
-
- Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow‐up study until 17 years old. Lancet 1995;346(8982):1065‐9. [PUBMED: 7564787] - PubMed
Saarinen 2000
-
- Saarinen KM, Savilahti E. Infant feeding patterns affect the subsequent immunological features in cow's milk allergy. Clinical & Experimental Allergy 2000;30(3):400‐6. [PUBMED: 10691899] - PubMed
Sampson 2004
Schultz Larsen 1996
-
- Schultz Larsen F. Atopic dermatitis: an increasing problem. Pediatric Allergy and Immunology 1996;7(9 Suppl):51‐3. [PUBMED: 9156729] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sears 1996
Sears 1998
-
- Sears MR. Evolution of asthma through childhood. Clinical & Experimental Allergy 1998;28(Suppl 5):82‐91. [PUBMED: 9988452] - PubMed
Sly 1999
Strachan 1996
Szajewska 2010
-
- Szajewska H, Horvath A. Meta‐analysis of the evidence for a partially hydrolyzed 100% whey formula for the prevention of allergic diseases. Current Medical Research and Opinion 2010;26:423‐37. - PubMed
References to other published versions of this review
Osborn 2003
Osborn 2006a
Osborn 2006b
Osborn 2017a
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

