Short, Tin-Free Synthesis of All Three Inthomycins
- PMID: 30338587
- PMCID: PMC6348375
- DOI: 10.1002/chem.201803794
Short, Tin-Free Synthesis of All Three Inthomycins
Abstract
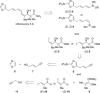
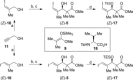
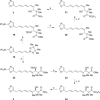
The inthomycins are a family of structurally and biologically rich natural products isolated from Streptomyces species. Herein the implementation of a modular synthetic route is reported that has enabled the enantioselective synthesis of all three inthomycins. Key steps include Suzuki and Sonogashira cross-couplings and an enantioselective Kiyooka aldol reaction.
Keywords: inthomycin; phthoxazolin; total synthesis.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Progress in the total synthesis of inthomycins.Beilstein J Org Chem. 2021 Jan 7;17:58-82. doi: 10.3762/bjoc.17.7. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33488832 Free PMC article. Review.
-
Asymmetric Total Synthesis of (-)-(3 R)-Inthomycin C.Org Lett. 2018 Jun 15;20(12):3583-3586. doi: 10.1021/acs.orglett.8b01370. Epub 2018 Jun 4. Org Lett. 2018. PMID: 29863350
-
Organocatalytic asymmetric syntheses of inthomycins A, B and C.Org Biomol Chem. 2012 Oct 28;10(40):8164-74. doi: 10.1039/c2ob26084k. Org Biomol Chem. 2012. PMID: 22971928
-
Asymmetric Total Synthesis of Inthomycins A, B, and C.J Org Chem. 2020 Apr 3;85(7):4795-4806. doi: 10.1021/acs.joc.0c00017. Epub 2020 Mar 24. J Org Chem. 2020. PMID: 32162914
-
Total synthesis of oxazolomycins.Chem Rec. 2014 Aug;14(4):663-77. doi: 10.1002/tcr.201402009. Epub 2014 Jul 28. Chem Rec. 2014. PMID: 25069829 Review.
Cited by
-
Kinetic resolution of racemic allylic alcohols via iridium-catalyzed asymmetric hydrogenation: scope, synthetic applications and insight into the origin of selectivity.Chem Sci. 2020 Dec 8;12(5):1937-1943. doi: 10.1039/d0sc05276k. Chem Sci. 2020. PMID: 34163958 Free PMC article.
-
The Implications of the Brønsted Acidic Properties of Crabtree-Type Catalysts in the Asymmetric Hydrogenation of Olefins.J Am Chem Soc. 2022 Sep 14;144(36):16252-16261. doi: 10.1021/jacs.2c07023. Epub 2022 Aug 31. J Am Chem Soc. 2022. PMID: 36044252 Free PMC article. Review.
-
Progress in the total synthesis of inthomycins.Beilstein J Org Chem. 2021 Jan 7;17:58-82. doi: 10.3762/bjoc.17.7. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33488832 Free PMC article. Review.
-
Oxazolomycins produced by Streptomyces glaucus and their cytotoxic activity.RSC Adv. 2021 Oct 28;11(55):35011-35019. doi: 10.1039/d1ra06182h. eCollection 2021 Oct 25. RSC Adv. 2021. PMID: 35494745 Free PMC article.
References
-
- Ömura S., Tanaka Y., Kanaya I., Shinose M., Takahashi Y., J. Antibiot. 1990, 43, 1034–1036. - PubMed
-
- Shiomi K., Arai N., Shinose M., Takahashi Y., Yoshida H., Iwabuchi J., Tanaka Y., Ōmura S., J. Antibiot. 1995, 48, 714–719. - PubMed
-
- Henkel T., Zeeck A., Liebigs Ann. Chem. 1991, 367–373.
-
- Tanaka Y., Kanaya I., Takahashi Y., Shinose M., Tanaka H., Ōmura S., J. Antibiot. 1993, 46, 1208–1213. - PubMed
-
- Ömura S., Gene 1992, 115, 141–149. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

