Mangiferin Potentiates Neuroprotection by Isoflurane in Neonatal Hypoxic Brain Injury by Reducing Oxidative Stress and Activation of Phosphatidylinositol-3-Kinase/Akt/Mammalian Target of Rapamycin (PI3K/Akt/mTOR) Signaling
- PMID: 30338764
- PMCID: PMC6354638
- DOI: 10.12659/MSM.908142
Mangiferin Potentiates Neuroprotection by Isoflurane in Neonatal Hypoxic Brain Injury by Reducing Oxidative Stress and Activation of Phosphatidylinositol-3-Kinase/Akt/Mammalian Target of Rapamycin (PI3K/Akt/mTOR) Signaling
Abstract
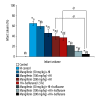
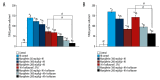
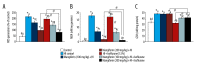
BACKGROUND Hypoxic-ischemic brain injury in the perinatal period is a main cause of perinatal mortality and neurologic complications in neonates and children. Recent studies have focused on the neuroprotective effect of anesthetic drugs. The volatile anesthetic isoflurane has been shown to exert neuroprotective effects in cerebral ischemia. Mangiferin is a natural polyphenol with various pharmacological properties, including antioxidant and ant-tumor effects. This study aimed to determine whether mangiferin potentiates the neuroprotective effects of isoflurane and also if mangiferin when administered alone exerts neuroprotective effects following hypoxic-ischemic brain injury. MATERIAL AND METHODS Sprague-Dawley rats were subjected to cerebral hypoxic ischemia on postnatal day 10 (P10). Mangiferin (50, 100, or 200 mg/kg b.w.) was intragastrically administered from P3 to P12 and 1 h prior to insult on the day of ischemic induction. At 3 h after hypoxia-ischemia (HI) insult, separate groups of rat pups were exposed to isoflurane (1.5%) for 6 h. Following 48 h of HI, the rats were sacrificed and brain tissues were used for analysis. RESULTS Mangiferin treatment attenuated neuronal apoptosis and reduced cerebral infarct volume. The expression of cleaved caspase-3 and apoptotic cascade proteins were regulated. The levels of reactive oxygen species (ROS) and malondialdehyde were reduced by mangiferin and/or isoflurane exposure. The levels of antioxidant glutathione were considerably raised under HI injury, which was modulated by mangiferin and isoflurane exposure. The PI3K/Akt signaling pathway, which was downregulated following HI insult, was activated by mangiferin and/or isoflurane. CONCLUSIONS This study reveals the potent neuroprotective efficacy of mangiferin against HI-induced brain injury via effectively modulating apoptotic pathways, ROS levels, and PI3K/Akt cascades while potentiating protective effects of isoflurane.
Conflict of interest statement
None.
Figures



References
-
- Vannucci RC, Connor JR, Mauger DT, et al. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55:158–63. - PubMed
-
- Volpe JJ. Perinatal brain injury: From pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. - PubMed
-
- Ferrieo DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. - PubMed
-
- Sasaoka N, Kawaguchi M, Kawaraguchi Y, et al. Isoflurane exerts a short-term but not a long-term preconditioning effect in neonatal rats exposed to a hypoxic-ischaemic neuronal injury. Acta Anaesthesiol Scand. 2009;53:46–54. - PubMed
-
- Ferriero DM, Bonifacio SL. The search continues for the elusive biomarkers of neonatal brain injury. J Pediatr. 2014;164:438–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

