Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species
- PMID: 30339218
- PMCID: PMC6300522
- DOI: 10.1093/femsre/fuy037
Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species
Abstract
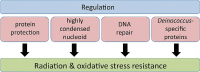
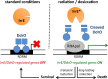
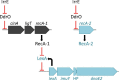
Deinococcus bacteria are famous for their extreme resistance to ionising radiation and other DNA damage- and oxidative stress-generating agents. More than a hundred genes have been reported to contribute to resistance to radiation, desiccation and/or oxidative stress in Deinococcus radiodurans. These encode proteins involved in DNA repair, oxidative stress defence, regulation and proteins of yet unknown function or with an extracytoplasmic location. Here, we analysed the conservation of radiation resistance-associated proteins in other radiation-resistant Deinococcus species. Strikingly, homologues of dozens of these proteins are absent in one or more Deinococcus species. For example, only a few Deinococcus-specific proteins and radiation resistance-associated regulatory proteins are present in each Deinococcus, notably the metallopeptidase/repressor pair IrrE/DdrO that controls the radiation/desiccation response regulon. Inversely, some Deinococcus species possess proteins that D. radiodurans lacks, including DNA repair proteins consisting of novel domain combinations, translesion polymerases, additional metalloregulators, redox-sensitive regulator SoxR and manganese-containing catalase. Moreover, the comparisons improved the characterisation of several proteins regarding important conserved residues, cellular location and possible protein-protein interactions. This comprehensive analysis indicates not only conservation but also large diversity in the molecular mechanisms involved in radiation resistance even within the Deinococcus genus.
Figures



References
-
- Agapov AA, Kulbachinskiy AV. Mechanisms of stress resistance and gene regulation in the radioresistant bacterium Deinococcus radiodurans. Biochemistry 2015;80:1201–16. - PubMed
-
- Agrawal R, Sahoo BK, Saini DK. Cross-talk and specificity in two-component signal transduction pathways. Future Microbiol 2016;11:685–97. - PubMed
-
- Anaganti N, Basu B, Mukhopadhyaya R et al. . Proximity of radiation desiccation response motif to the core promoter is essential for basal repression as well as gamma radiation-induced gyrB gene expression in Deinococcus radiodurans. Gene 2017;615:8–17. - PubMed

