Assembly and repair of eye-to-brain connections
- PMID: 30339988
- PMCID: PMC6504177
- DOI: 10.1016/j.conb.2018.10.001
Assembly and repair of eye-to-brain connections
Abstract
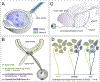
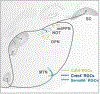
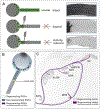
Vision is the sense humans rely on most to navigate the world and survive. A tremendous amount of research has focused on understanding the neural circuits for vision and the developmental mechanisms that establish them. The eye-to-brain, or 'retinofugal' pathway remains a particularly important model in these contexts because it is essential for sight, its overt anatomical features relate to distinct functional attributes and those features develop in a tractable sequence. Much progress has been made in understanding the growth of retinal axons out of the eye, their selection of targets in the brain, the development of laminar and cell type-specific connectivity within those targets, and also dendritic connectivity within the retina itself. Moreover, because the retinofugal pathway is prone to degeneration in many common blinding diseases, understanding the cellular and molecular mechanisms that establish connectivity early in life stands to provide valuable insights into approaches that re-wire this pathway after damage or loss. Here we review recent progress in understanding the development of retinofugal pathways and how this information is important for improving visual circuit regeneration.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

References
-
- Ramón y Cajal S. 1995. Histology of the nervous system of man and vertebrates New York: Oxford University Press.
-
- Ramon y Cajal S. 1928. Degeneration and Regeneration of the Nervous System New York: Oxford University Press, Reprinted 1991.
-
-
Baden T, Berens P, Franke K, Rosόn MR, Bethge M, Euler T. 2016. The functional diversity of retinal ganglion cells in the mouse. Nature 529: 345–50.
A comprehensive exploration of the functional types of RGCs in mouse retina, achieved by calcium imaging all the RGCs in a large patch of explanted retina. A technical tour-de-force, this approach expanded beyond targeted recordings and previous anatomical studies in mouse.
-
-
-
Roska B, Werblin F. 2001. Vertical interactions across ten parallel stacked representations in the mammalian retina. Nature 410: 583–587.
Using patch-clamp recordings from whole-mount rabbit retinas, the authors show that the inner plexiform layer is organized into at least ten parallel stacks of strata that all different RGCs to receive distinct amacrine and bipolar inputs. These neural inputs act in a distinct spatial and temporal fashion and are mediated by feedback inhibition, indicating that the interactions between strata occur in a vertical fashion.
-
-
-
Morin LP, Studholme KM. 2014. Retinofugal projections in the mouse. J. Comp. Neurol 522: 3733–53.
This is the most comprehensive paper to date on the number, location and pattern of retinorecipient targets in mouse. Using intraocular injections of cholera-toxin β subunit, the authors compare and contrast the visual projections in the mouse with that of the hamster and the rat.
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

