Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer
- PMID: 30340040
- PMCID: PMC6232850
- DOI: 10.1016/j.cell.2018.09.004
Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer
Abstract
Dietary soluble fibers are fermented by gut bacteria into short-chain fatty acids (SCFA), which are considered broadly health-promoting. Accordingly, consumption of such fibers ameliorates metabolic syndrome. However, incorporating soluble fiber inulin, but not insoluble fiber, into a compositionally defined diet, induced icteric hepatocellular carcinoma (HCC). Such HCC was microbiota-dependent and observed in multiple strains of dysbiotic mice but not in germ-free nor antibiotics-treated mice. Furthermore, consumption of an inulin-enriched high-fat diet induced both dysbiosis and HCC in wild-type (WT) mice. Inulin-induced HCC progressed via early onset of cholestasis, hepatocyte death, followed by neutrophilic inflammation in liver. Pharmacologic inhibition of fermentation or depletion of fermenting bacteria markedly reduced intestinal SCFA and prevented HCC. Intervening with cholestyramine to prevent reabsorption of bile acids also conferred protection against such HCC. Thus, its benefits notwithstanding, enrichment of foods with fermentable fiber should be approached with great caution as it may increase risk of HCC.
Keywords: bile acids.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
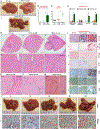




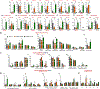

Comment in
-
When Soluble Fibers Meet Hepatocellular Carcinoma: The Dark Side of Fermentation.Cell Metab. 2018 Nov 6;28(5):673-675. doi: 10.1016/j.cmet.2018.10.009. Cell Metab. 2018. PMID: 30403986
-
Precision dietary supplementation based on personal gut microbiota.Nat Rev Gastroenterol Hepatol. 2019 Apr;16(4):204-206. doi: 10.1038/s41575-019-0108-z. Nat Rev Gastroenterol Hepatol. 2019. PMID: 30644454 Free PMC article.
-
Soluble Fibers Improve Metabolic Syndrome but May Cause Liver Disease and Hepatocellular Carcinoma.Hepatology. 2019 Aug;70(2):739-741. doi: 10.1002/hep.30565. Epub 2019 Apr 11. Hepatology. 2019. PMID: 30762890 No abstract available.
-
Commentary: Dysregulated Microbial Fermentation of Soluble Fiber Induces Cholestatic Liver Cancer.Front Cell Infect Microbiol. 2019 May 21;9:155. doi: 10.3389/fcimb.2019.00155. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31231615 Free PMC article. No abstract available.
References
-
- Asimakopoulou A, Borkham-Kamphorst E, Tacke F, and Weiskirchen R (2016). Lipocalin-2 (NGAL/LCN2), a “help-me” signal in organ inflammation. Hepatology 63, 669–671. - PubMed
-
- Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, Moriyama EH, Copeland JK, Surendra A, Kumar S, et al. (2014). Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 158, 288–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

