Determinants of Polar versus Nematic Organization in Networks of Dynamic Microtubules and Mitotic Motors
- PMID: 30340043
- PMCID: PMC6198040
- DOI: 10.1016/j.cell.2018.09.029
Determinants of Polar versus Nematic Organization in Networks of Dynamic Microtubules and Mitotic Motors
Abstract
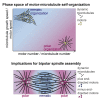
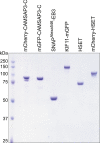
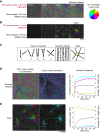
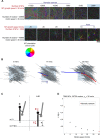
During cell division, mitotic motors organize microtubules in the bipolar spindle into either polar arrays at the spindle poles or a "nematic" network of aligned microtubules at the spindle center. The reasons for the distinct self-organizing capacities of dynamic microtubules and different motors are not understood. Using in vitro reconstitution experiments and computer simulations, we show that the human mitotic motors kinesin-5 KIF11 and kinesin-14 HSET, despite opposite directionalities, can both organize dynamic microtubules into either polar or nematic networks. We show that in addition to the motor properties the natural asymmetry between microtubule plus- and minus-end growth critically contributes to the organizational potential of the motors. We identify two control parameters that capture system composition and kinetic properties and predict the outcome of microtubule network organization. These results elucidate a fundamental design principle of spindle bipolarity and establish general rules for active filament network organization.
Keywords: Cytosim; active network; computer simulation; cytoskeleton; in vitro reconsititution; kinesin; microtubule; molecular motor; self-organization; spindle assembly.
Copyright © 2018 The Francis Crick Institute. Published by Elsevier Inc. All rights reserved.
Figures










Similar articles
-
Molecular design principles for bipolar spindle organization by two opposing motors.Proc Natl Acad Sci U S A. 2025 Mar 25;122(12):e2422190122. doi: 10.1073/pnas.2422190122. Epub 2025 Mar 21. Proc Natl Acad Sci U S A. 2025. PMID: 40117309 Free PMC article.
-
Cross-linker design determines microtubule network organization by opposing motors.Proc Natl Acad Sci U S A. 2022 Aug 16;119(33):e2206398119. doi: 10.1073/pnas.2206398119. Epub 2022 Aug 12. Proc Natl Acad Sci U S A. 2022. PMID: 35960844 Free PMC article.
-
Minus-end-directed Kinesin-14 motors align antiparallel microtubules to control metaphase spindle length.Dev Cell. 2014 Oct 13;31(1):61-72. doi: 10.1016/j.devcel.2014.07.023. Dev Cell. 2014. PMID: 25313961 Free PMC article.
-
Mechanisms by Which Kinesin-5 Motors Perform Their Multiple Intracellular Functions.Int J Mol Sci. 2021 Jun 15;22(12):6420. doi: 10.3390/ijms22126420. Int J Mol Sci. 2021. PMID: 34203964 Free PMC article. Review.
-
Mitotic functions of kinesin-5.Semin Cell Dev Biol. 2010 May;21(3):255-9. doi: 10.1016/j.semcdb.2010.01.019. Epub 2010 Jan 28. Semin Cell Dev Biol. 2010. PMID: 20109572 Free PMC article. Review.
Cited by
-
Active forces shape the metaphase spindle through a mechanical instability.Proc Natl Acad Sci U S A. 2020 Jul 14;117(28):16154-16159. doi: 10.1073/pnas.2002446117. Epub 2020 Jun 29. Proc Natl Acad Sci U S A. 2020. PMID: 32601228 Free PMC article.
-
The ATM and ATR kinases regulate centrosome clustering and tumor recurrence by targeting KIFC1 phosphorylation.Nat Commun. 2021 Jan 4;12(1):20. doi: 10.1038/s41467-020-20208-x. Nat Commun. 2021. PMID: 33397932 Free PMC article.
-
Force balance of opposing diffusive motors generates polarity-sorted microtubule patterns.Proc Natl Acad Sci U S A. 2024 Dec 3;121(49):e2406985121. doi: 10.1073/pnas.2406985121. Epub 2024 Nov 26. Proc Natl Acad Sci U S A. 2024. PMID: 39589887 Free PMC article.
-
NuMA mechanically reinforces the spindle independently of its partner dynein.Curr Biol. 2025 Jul 30:S0960-9822(25)00894-2. doi: 10.1016/j.cub.2025.07.028. Online ahead of print. Curr Biol. 2025. PMID: 40769151
-
Effects of spatial dimensionality and steric interactions on microtubule-motor self-organization.Phys Biol. 2019 Apr 23;16(4):046004. doi: 10.1088/1478-3975/ab0fb1. Phys Biol. 2019. PMID: 31013252 Free PMC article.
References
-
- Aguirre-Portolés C., Bird A.W., Hyman A., Cañamero M., Pérez de Castro I., Malumbres M. Tpx2 controls spindle integrity, genome stability, and tumor development. Cancer Res. 2012;72:1518–1528. - PubMed
-
- Alvarado J., Sheinman M., Sharma A., MacKintosh F.C., Koenderink G.H. Force percolation of contractile active gels. Soft Matter. 2017;13:5624–5644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

