p73 Is Required for Ovarian Follicle Development and Regulates a Gene Network Involved in Cell-to-Cell Adhesion
- PMID: 30340069
- PMCID: PMC6197761
- DOI: 10.1016/j.isci.2018.09.018
p73 Is Required for Ovarian Follicle Development and Regulates a Gene Network Involved in Cell-to-Cell Adhesion
Abstract
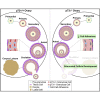
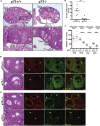
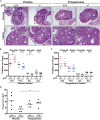
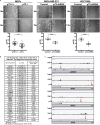
We report that p73 is expressed in ovarian granulosa cells and that loss of p73 leads to attenuated follicle development, ovulation, and corpus luteum formation, resulting in decreased levels of circulating progesterone and defects in mammary gland branching. Ectopic progesterone in p73-deficient mice completely rescued the mammary branching and partially rescued the ovarian follicle development defects. Performing RNA sequencing (RNA-seq) on transcripts from murine wild-type and p73-deficient antral follicles, we discovered differentially expressed genes that regulate biological adhesion programs. Through modulation of p73 expression in murine granulosa cells and transformed cell lines, followed by RNA-seq and chromatin immunoprecipitation sequencing, we discovered p73-dependent regulation of a gene set necessary for cell adhesion and migration and components of the focimatrix (focal intra-epithelial matrix), a basal lamina between granulosa cells that promotes follicle maturation. In summary, p73 is essential for ovarian folliculogenesis and functions as a key regulator of a gene network involved in cell-to-cell adhesion and migration.
Keywords: Developmental Biology; Functional Aspects of Cell Biology; Molecular Network; Omics.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Bae W.K., Hong C.S., Park M.R., Sun E.G., Lee J.H., Kang K., Ryu K.H., Shim H.J., Hwang J.E., Cho S.H., Chung I.J. TAp73 inhibits cell invasion and migration by directly activating KAI1 expression in colorectal carcinoma. Cancer Lett. 2018;415:106–116. - PubMed
-
- Barbareschi M., Pecciarini L., Cangi M.G., Macri E., Rizzo A., Viale G., Doglioni C. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am. J. Surg. Pathol. 2001;25:1054–1060. - PubMed
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- S10 OD016355/OD/NIH HHS/United States
- R01 CA105436/CA/NCI NIH HHS/United States
- U2C DK059637/DK/NIDDK NIH HHS/United States
- U54 HD028934/HD/NICHD NIH HHS/United States
- P50 HD028934/HD/NICHD NIH HHS/United States
- P30 HD028934/HD/NICHD NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- P50 CA098131/CA/NCI NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

