Practical and clinical utility of non-invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: a post hoc analysis of the randomized, sham-controlled, double-blind PRESTO trial
- PMID: 30340460
- PMCID: PMC6742918
- DOI: 10.1186/s10194-018-0928-1
Practical and clinical utility of non-invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: a post hoc analysis of the randomized, sham-controlled, double-blind PRESTO trial
Erratum in
-
Correction to: Practical and clinical utility of non-invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: a post hoc analysis of the randomized, sham-controlled, double-blind PRESTO trial.J Headache Pain. 2019 Jan 7;20(1):1. doi: 10.1186/s10194-018-0948-x. J Headache Pain. 2019. PMID: 30616570 Free PMC article.
Abstract
Background: The PRESTO study of non-invasive vagus nerve stimulation (nVNS; gammaCore®) featured key primary and secondary end points recommended by the International Headache Society to provide Class I evidence that for patients with an episodic migraine, nVNS significantly increases the probability of having mild pain or being pain-free 2 h post stimulation. Here, we examined additional data from PRESTO to provide further insights into the practical utility of nVNS by evaluating its ability to consistently deliver clinically meaningful improvements in pain intensity while reducing the need for rescue medication.
Methods: Patients recorded pain intensity for treated migraine attacks on a 4-point scale. Data were examined to compare nVNS and sham with regard to the percentage of patients who benefited by at least 1 point in pain intensity. We also assessed the percentage of attacks that required rescue medication and pain-free rates stratified by pain intensity at treatment initiation.
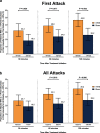
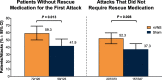
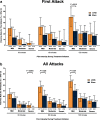
Results: A significantly higher percentage of patients who used acute nVNS treatment (n = 120) vs sham (n = 123) reported a ≥ 1-point decrease in pain intensity at 30 min (nVNS, 32.2%; sham, 18.5%; P = 0.020), 60 min (nVNS, 38.8%; sham, 24.0%; P = 0.017), and 120 min (nVNS, 46.8%; sham, 26.2%; P = 0.002) after the first attack. Similar significant results were seen when assessing the benefit in all attacks. The proportion of patients who did not require rescue medication was significantly higher with nVNS than with sham for the first attack (nVNS, 59.3%; sham, 41.9%; P = 0.013) and all attacks (nVNS, 52.3%; sham, 37.3%; P = 0.008). When initial pain intensity was mild, the percentage of patients with no pain after treatment was significantly higher with nVNS than with sham at 60 min (all attacks: nVNS, 37.0%; sham, 21.2%; P = 0.025) and 120 min (first attack: nVNS, 50.0%; sham, 25.0%; P = 0.018; all attacks: nVNS, 46.7%; sham, 30.1%; P = 0.037).
Conclusions: This post hoc analysis demonstrated that acute nVNS treatment quickly and consistently reduced pain intensity while decreasing rescue medication use. These clinical benefits provide guidance in the optimal use of nVNS in everyday practice, which can potentially reduce use of acute pharmacologic medications and their associated adverse events.
Trial registration: ClinicalTrials.gov identifier: NCT02686034 .
Keywords: Migraine; Neuromodulation; Pain intensity; Post hoc analysis; Rescue medication; Vagus nerve stimulation.
Conflict of interest statement
Figures


References
-
- Kinfe TM, Pintea B, Muhammad S, Zaremba S, Roeske S, Simon BJ, Vatter H. Cervical non-invasive vagus nerve stimulation (nVNS) for preventive and acute treatment of episodic and chronic migraine and migraine-associated sleep disturbance: a prospective observational cohort study. J Headache Pain. 2015;16:101. doi: 10.1186/s10194-015-0582-9. - DOI - PMC - PubMed
-
- Tassorelli C, Grazzi L, de Tommaso M, Pierangeli G, Martelletti P, Rainero I, Dorlas S, Geppetti P, Ambrosini A, Sarchielli P, Liebler E, Barbanti P, Group PS Noninvasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology. 2018;91(4):e364–e373. doi: 10.1212/WNL.0000000000005857. - DOI - PMC - PubMed
-
- Buse DC, Serrano D, Reed ML, Kori SH, Cunanan CM, Adams AM, Lipton RB. Adding additional acute medications to a triptan regimen for migraine and observed changes in headache-related disability: results from the American migraine prevalence and prevention (AMPP) study. Headache. 2015;55(6):825–839. doi: 10.1111/head.12556. - DOI - PubMed

