Salicylic acid reverses pollen abortion of rice caused by heat stress
- PMID: 30340520
- PMCID: PMC6194599
- DOI: 10.1186/s12870-018-1472-5
Salicylic acid reverses pollen abortion of rice caused by heat stress
Abstract
Background: Extremely high temperatures are becoming an increasingly severe threat to crop yields. It is well documented that salicylic acid (SA) can enhance the stress tolerance of plants; however, its effect on the reproductive organs of rice plants has not been described before. To investigate the mechanism underlying the SA-mediated alleviation of the heat stress damage to rice pollen viability, a susceptible cultivar (Changyou1) was treated with SA at the pollen mother cell (PMC) meiosis stage and then subjected to heat stress of 40 °C for 10 d until 1d before flowering.
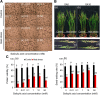
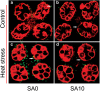
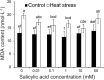
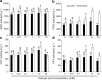
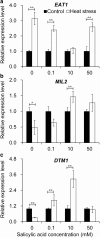
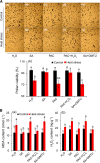
Results: Under control conditions, no significant difference was found in pollen viability and seed-setting rate in SA treatments. However, under heat stress conditions, SA decreased the accumulation of reactive oxygen species (ROS) in anthers to prevent tapetum programmed cell death (PCD) and degradation. The genes related to tapetum development, such as EAT1 (Eternal Tapetum 1), MIL2 (Microsporeless 2), and DTM1 (Defective Tapetum and Meiocytese 1), were found to be involved in this process. When rice plants were exogenously sprayed with SA or paclobutrazol (PAC, a SA inhibitor) + H2O2 under heat stress, a significantly higher pollen viability was found compared to plants sprayed with H2O, PAC, or SA + dimethylthiourea (DMTU, an H2O2 and OH· scavenger). Additionally, a sharp increase in H2O2 was observed in the SA or PAC+ H2O2 treatment groups compared to other treatments.
Conclusion: We suggest that H2O2 may play an important role in mediating SA to prevent pollen abortion caused by heat stress through inhibiting the tapetum PCD.
Keywords: H2O2; Heat stress; Oryza sativa L.; Pollen viability; Salicylic acid; Tapetum.
Conflict of interest statement
Ethics approval and consent to participate
The rice seed, Changyou1, is very a common and broadly cultivated variety in China. The seed was bought from the academy of agriculture sciences of Changshu city, Jiangsu province. Our present work didn’t use transgenic technology or material therefore it does not require ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Bheemanahalli R, Sathishraj R, Tack J, Nalley LL, Muthurajan R, Jagadish KSV. Temperature thresholds for spikelet sterility and associated warming impacts for sub-tropical rice. Agric For Meteorol. 2016;221:122–130. doi: 10.1016/j.agrformet.2016.02.003. - DOI
-
- Sathishraj R, Bheemanahalli R, Ramachandran M, Dingkuhn M, Muthurajan R, Krishna JSV. Capturing heat stress induced variability in spikelet sterility using panicle, leaf and air temperature under field conditions. Field Crop Res. 2016;190:10–17. doi: 10.1016/j.fcr.2015.10.012. - DOI
-
- Fu GF, Zhang CX, Yang XQ, Yang YJ, Chen TT, Zhao X, Fu WM, Feng BH, Zhang XF, Tao LX, Jin QY. Action mechanism by which SA alleviates high temperature induced inhibition to spikelet differentiation. Chin J Rice Sci. 2015;29:637–647.
-
- Zhang CX, Feng BH, Chen TT, Fu WM, Li HB, Li GY, Jin QY, Tao LX, Fu GF. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ Exp Bot. 2018;155:718–733. doi: 10.1016/j.envexpbot.2018.08.021. - DOI
-
- Fu GF, Song J, Xiong J, Liao XY, Zhang XF, Wang X, Le MK, Tao LX. Thermal resistance of common rice maintainer and restorer lines to high temperature during flowering and early grain filling stages. Rice Sci. 2012;19:309–314. doi: 10.1016/S1672-6308(12)60055-9. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

