Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier
- PMID: 30340614
- PMCID: PMC6194691
- DOI: 10.1186/s12987-018-0113-6
Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier
Abstract
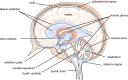
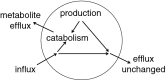
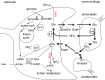
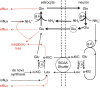
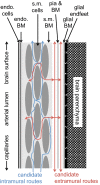
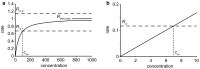
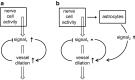
This review considers efflux of substances from brain parenchyma quantified as values of clearances (CL, stated in µL g-1 min-1). Total clearance of a substance is the sum of clearance values for all available routes including perivascular pathways and the blood-brain barrier. Perivascular efflux contributes to the clearance of all water-soluble substances. Substances leaving via the perivascular routes may enter cerebrospinal fluid (CSF) or lymph. These routes are also involved in entry to the parenchyma from CSF. However, evidence demonstrating net fluid flow inwards along arteries and then outwards along veins (the glymphatic hypothesis) is still lacking. CLperivascular, that via perivascular routes, has been measured by following the fate of exogenously applied labelled tracer amounts of sucrose, inulin or serum albumin, which are not metabolized or eliminated across the blood-brain barrier. With these substances values of total CL ≅ 1 have been measured. Substances that are eliminated at least partly by other routes, i.e. across the blood-brain barrier, have higher total CL values. Substances crossing the blood-brain barrier may do so by passive, non-specific means with CLblood-brain barrier values ranging from < 0.01 for inulin to > 1000 for water and CO2. CLblood-brain barrier values for many small solutes are predictable from their oil/water partition and molecular weight. Transporters specific for glucose, lactate and many polar substrates facilitate efflux across the blood-brain barrier producing CLblood-brain barrier values > 50. The principal route for movement of Na+ and Cl- ions across the blood-brain barrier is probably paracellular through tight junctions between the brain endothelial cells producing CLblood-brain barrier values ~ 1. There are large fluxes of amino acids into and out of the brain across the blood-brain barrier but only small net fluxes have been observed suggesting substantial reuse of essential amino acids and α-ketoacids within the brain. Amyloid-β efflux, which is measurably faster than efflux of inulin, is primarily across the blood-brain barrier. Amyloid-β also leaves the brain parenchyma via perivascular efflux and this may be important as the route by which amyloid-β reaches arterial walls resulting in cerebral amyloid angiopathy.
Keywords: ABC transporters; Amino acid metabolism; Basement membrane; Blood–brain barrier permeability; Branched chain amino acid shuttle; Carrier mechanism; Diffusion; Efflux rate constant; Linear free energy relations; Perivascular convection; SLC transporters; Trans-stimulation; Transcytosis; Volume of distribution.
Figures


















References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

